Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis
- PMID: 14681931
- PMCID: PMC3275427
- DOI: 10.1002/cne.10995
Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis
Abstract
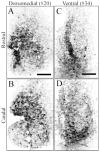
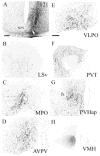
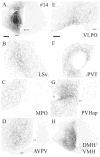
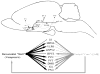
Circadian rhythms in physiology and behavior are controlled by pacemaker cells located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The mammalian SCN can be classified into two subdivisions (core and shell) based on the organization of neuroactive substances, inputs, and outputs. Recent studies in our laboratory indicate that these subdivisions are associated with functional specialization in Syrian hamsters. The core region, marked by calbindin-D(28K) (CalB)-containing cells, expresses light-induced, but not rhythmic, clock genes. In the shell compartment, marked by vasopressinergic cells and fibers, clock gene expression is rhythmic. Given these findings, an important question is how photic and rhythmic information are integrated and communicated from each of these regions to effector areas. The present study used localized, intra-SCN iontophoretic injections of the anterograde tracer biotinylated dextran amine (BDA) to investigate intra-SCN connectivity and the neural pathways by which information is communicated from SCN subregions to targets. Intra-SCN connections project from the core to the shell compartment of the SCN, but not from the shell to the CalB region of the SCN. Retrograde tracing experiments were performed using cholera toxin-beta (CTB) to determine more specifically whether SCN efferents originated in the core or shell using neurochemical markers for the rhythmic (vasopressin) and light-induced (CalB) SCN subregions. The combined results from anterograde and retrograde experiments suggest that all SCN targets receive information from both the light-induced and rhythmic regions of the SCN (albeit to varying degrees) and indicate that light and rhythmic information may be integrated both within the SCN and at target effector areas.
Copyright 2003 Wiley-Liss, Inc.
Figures










Similar articles
-
Targeted microlesions reveal novel organization of the hamster suprachiasmatic nucleus.J Neurosci. 2004 Mar 10;24(10):2449-57. doi: 10.1523/JNEUROSCI.5323-03.2004. J Neurosci. 2004. PMID: 15014120 Free PMC article.
-
Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: a dual tract-tracing study in rat.Eur J Neurosci. 2002 Oct;16(7):1195-213. doi: 10.1046/j.1460-9568.2002.02196.x. Eur J Neurosci. 2002. PMID: 12405980
-
Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state.Neuroscience. 2005;130(1):165-83. doi: 10.1016/j.neuroscience.2004.08.030. Neuroscience. 2005. PMID: 15561433
-
The suprachiasmatic nucleus: a clock of multiple components.J Biol Rhythms. 2003 Dec;18(6):435-49. doi: 10.1177/0748730403259106. J Biol Rhythms. 2003. PMID: 14667145 Review.
-
Serotonergic innervation of the hypothalamic suprachiasmatic nucleus and photic regulation of circadian rhythms.Biol Cell. 1997 Nov;89(8):513-23. doi: 10.1016/s0248-4900(98)80007-5. Biol Cell. 1997. PMID: 9618901 Review.
Cited by
-
Circadian regulation of kisspeptin in female reproductive functioning.Adv Exp Med Biol. 2013;784:385-410. doi: 10.1007/978-1-4614-6199-9_18. Adv Exp Med Biol. 2013. PMID: 23550016 Free PMC article. Review.
-
Suppression of Circadian Timing and Its Impact on the Hippocampus.Front Neurosci. 2021 Apr 8;15:642376. doi: 10.3389/fnins.2021.642376. eCollection 2021. Front Neurosci. 2021. PMID: 33897354 Free PMC article. Review.
-
Suprachiasmatic function in a circadian period mutant: Duper alters light-induced activation of vasoactive intestinal peptide cells and PERIOD1 immunostaining.Eur J Neurosci. 2018 Dec;48(11):3319-3334. doi: 10.1111/ejn.14214. Eur J Neurosci. 2018. PMID: 30346078 Free PMC article.
-
Intrinsic regulation of spatiotemporal organization within the suprachiasmatic nucleus.PLoS One. 2011 Jan 7;6(1):e15869. doi: 10.1371/journal.pone.0015869. PLoS One. 2011. PMID: 21249213 Free PMC article.
-
Ultrastructure of Synaptic Connectivity within Subregions of the Suprachiasmatic Nucleus Revealed by a Genetically Encoded Tag and Serial Blockface Electron Microscopy.eNeuro. 2023 Aug 23;10(8):ENEURO.0227-23.2023. doi: 10.1523/ENEURO.0227-23.2023. Print 2023 Aug. eNeuro. 2023. PMID: 37500494 Free PMC article.
References
-
- Ambrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. - PubMed
-
- Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythms. 2001;16:196–204. - PubMed
-
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–299. - PubMed
-
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–1578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

