Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production
- PMID: 14684838
- PMCID: PMC316310
- DOI: 10.1104/pp.103.028712
Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production
Abstract
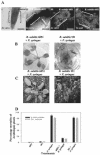
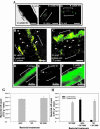
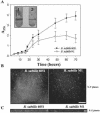
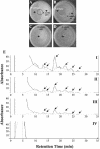
Relatively little is known about the exact mechanisms used by Bacillus subtilis in its behavior as a biocontrol agent on plants. Here, we report the development of a sensitive plant infection model demonstrating that the bacterial pathogen Pseudomonas syringae pv tomato DC3000 is capable of infecting Arabidopsis roots both in vitro and in soil. Using this infection model, we demonstrated the biocontrol ability of a wild-type B. subtilis strain 6051 against P. syringae. Arabidopsis root surfaces treated with B. subtilis were analyzed with confocal scanning laser microscopy to reveal a three-dimensional B. subtilis biofilm. It is known that formation of biofilms by B. subtilis is a complex process that includes secretion of surfactin, a lipopeptide antimicrobial agent. To determine the role of surfactin in biocontrol by B. subtilis, we tested a mutant strain, M1, with a deletion in a surfactin synthase gene and, thus, deficient in surfactin production. B. subtilis M1 was ineffective as a biocontrol agent against P. syringae infectivity in Arabidopsis and also failed to form robust biofilms on either roots or inert surfaces. The antibacterial activity of surfactin against P. syringae was determined in both broth and agar cultures and also by live-dead staining methods. Although the minimum inhibitory concentrations determined were relatively high (25 microg mL(-1)), the levels of the lipopeptide in roots colonized by B. subtilis are likely to be sufficient to kill P. syringae. Our results collectively indicate that upon root colonization, B. subtilis 6051 forms a stable, extensive biofilm and secretes surfactin, which act together to protect plants against attack by pathogenic bacteria.
Figures

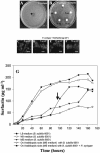
References
-
- Bianciotto V, Andreotti S, Balestrini R, Bonfante R, Perotto S (2001) Mucoid mutants of the biocontrol strain Pseudomonas fluorescens CHA0 show increased ability in biofilm formation on mycorrhizal and nonmycorrhizal carrot roots. Mol Plant-Microbe Interact 14: 255-260 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

