Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP
- PMID: 14684870
- PMCID: PMC6740956
- DOI: 10.1523/JNEUROSCI.23-37-11681.2003
Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP
Abstract
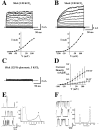
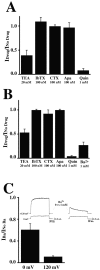
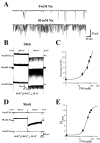
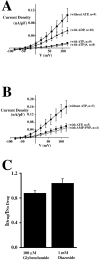
Neuronal stressors such as hypoxia and firing of action potentials at very high frequencies cause intracellular Na+ to rise and ATP to be consumed faster than it can be regenerated. We report the cloning of a gene encoding a K+ channel, Slick, and demonstrate that functionally it is a hybrid between two classes of K+ channels, Na+-activated (KNa) and ATP-sensitive (KATP) K+ channels. The Slick channel is activated by intracellular Na+ and Cl- and is inhibited by intracellular ATP. Slick is widely expressed in the CNS and is detected in heart. We identify a consensus ATP binding site near the C terminus of the channel that is required for ATP and its nonhydrolyzable analogs to reduce open probability. The convergence of Na+, Cl-, and ATP sensitivity in one channel may endow Slick with the ability to integrate multiple indicators of the metabolic state of a cell and to adjust electrical activity appropriately.
Figures



References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ ( 1990) Basic local alignment search tool. J Mol Biol 215: 403-410. - PubMed
-
- Bader CR, Bernheim L, Bertrand D ( 1985) Sodium-activated potassium current in cultured avian neurons. Nature 317: 540-542. - PubMed
-
- Bennington JH, Heller HC ( 1995) Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol 45: 347-360. - PubMed
-
- Bertrand D, Bader CR, Berheim L, Haimann ( 1989) KNa, A sodium activated potassium current. Pflügers Arch 414: S76-S79. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
