Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis
- PMID: 14684878
- PMCID: PMC6740953
- DOI: 10.1523/JNEUROSCI.23-37-11759.2003
Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis
Abstract
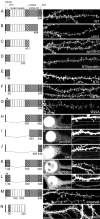
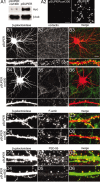
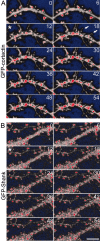
The number and shape of dendritic spines are influenced by activity and regulated by molecules that organize the actin cytoskeleton of spines. Cortactin is an F-actin binding protein and activator of the Arp2/3 actin nucleation machinery that also interacts with the postsynaptic density (PSD) protein Shank. Cortactin is concentrated in dendritic spines of cultured hippocampal neurons, and the N-terminal half of the protein containing the Arp2/3 and F-actin binding domains is necessary and sufficient for spine targeting. Knockdown of cortactin protein by short-interfering RNA (siRNA) results in depletion of dendritic spines in hippocampal neurons, whereas overexpression of cortactin causes elongation of spines. In response to synaptic stimulation and NMDA receptor activation, cortactin redistributes rapidly from spines to dendritic shaft, correlating with remodeling of the actin cytoskeleton, implicating cortactin in the activity-dependent regulation of spine morphogenesis.
Figures




References
-
- Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky LM, Segall JE, Condeelis J ( 2001) The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol 11: 620-625. - PubMed
-
- Benson DL, Watkins FH, Steward O, Banker G ( 1994) Characterization of GABAergic neurons in hippocampal cell cultures. J Neurocytol 23: 279-295. - PubMed
-
- Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG ( 2002) Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 26: 453-464. - PubMed
-
- Brewer GJ, Torricelli JR, Evege EK, Price PJ ( 1993) Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res 35: 567-576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
