What kinesin does at roadblocks: the coordination mechanism for molecular walking
- PMID: 14685258
- PMCID: PMC1271674
- DOI: 10.1038/sj.emboj.7600042
What kinesin does at roadblocks: the coordination mechanism for molecular walking
Abstract
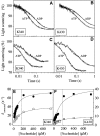
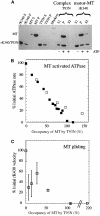
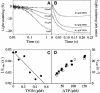
Competing models for the coordination of processive stepping in kinesin can be tested by introducing a roadblock to prevent lead head attachment. We used T93N, an irreversibly binding mutant monomer, as a roadblock, and measured the rates of nucleotide-induced detachment of kinesin monomers or dimers with and without the T93N roadblock using microflash photolysis combined with stopped flow. Control nucleotide-induced monomer (rK340) unbinding was 73.6 s(-1) for ATP and 40.5 s(-1) for ADP. Control ADP-induced dimer (rK430) unbinding was 18.6 s(-1). Added 20 mM Pi slowed both monomer and dimer unbinding. With the roadblock in place, lead head attachment of dimers is prevented and ATP-induced trail head unbinding was then 42 s(-1). This is less than two-fold slower than the stepping rate of unimpeded rK430 dimers (50-70 s(-1)), indicating that during walking, lead head attachment induces at most only a slight (less than two-fold) acceleration of trail head detachment. As we discuss, this implies a coordination model having very fast (>2000 s(-1)) ATP-induced attachment of the lead head, followed by slower, strain-sensitive ADP release from the lead head.
Figures


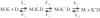
References
-
- Case RB, Rice S, Hart CL, Ly B, Vale RD (2000) Role of the kinesin neck linker and catalytic core in microtubule-based motility [see comments]. Curr Biol 10: 157–160 - PubMed
-
- Coy DL, Wagenbach M, Howard J (1999) Kinesin takes one 8-nm step for each ATP that it hydrolyzes. J Biol Chem 274: 3667–3671 - PubMed
-
- Crevel IM, Lockhart A, Cross RA (1996) Weak and strong states of kinesin and ncd. J Mol Biol 257: 66–76 - PubMed
-
- Crevel IM, Lockhart A, Cross RA (1997) Kinetic evidence for low chemical processivity in ncd and Eg5. J Mol Biol 273: 160–170 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

