Chaperokine-induced signal transduction pathways
- PMID: 14686091
- PMCID: PMC1822330
Chaperokine-induced signal transduction pathways
Abstract
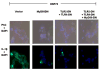
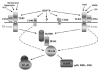
A turning point in understanding the function of heat shock proteins (HSP) on components of the immune system has now begun. From their original description as intracellular molecular chaperones of naïve, aberrantly folded or mutated proteins and primarily involved in cytoprotection in response to stressful stimuli, in recent years, new functions of HSP have been revealed. Strong evidence now exists demonstrating that the seventy-kDa heat shock protein (HSP70) exits mammalian cells not only following necrotic cell death but also by a process involving its active release in response to stresses including cytokines, acute psychological stress and exercise. The released extracellular HSP70 interacts with cells of the immune system and exerts immunoregulatory effects--known as the chaperokine activity of HSP70. The chaperokine activity of HSP70 is mediated in part by utilizing surface receptors for both Toll-like receptor-2 (TLR2; receptor for Gram-positive bacteria) and TLR4 (receptor for Gram-negative bacteria) in a CD14-dependent fashion. These findings suggest an important role for heat shock proteins in host protection against pathogenic infection. This review will briefly discuss chaperokine-induced signaling and its relevance to infection and exercise.
Figures
References
-
- Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000;12:13–19. - PubMed
-
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee H-G, de la Salle H, Schild H. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. - PubMed
-
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. - PubMed
-
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
