Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae
- PMID: 14688087
- PMCID: PMC343998
- DOI: 10.1128/IAI.72.1.106-113.2004
Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae
Abstract
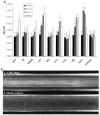
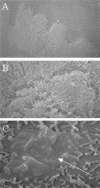
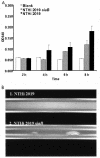
Nontypeable Haemophilus influenzae (NTHi) is a major cause of opportunistic respiratory tract infections, including otitis media and bronchitis. The persistence of NTHi in vivo is thought to involve bacterial persistence in a biofilm community. Therefore, there is a need for further definition of bacterial factors contributing to biofilm formation by NTHi. Like other bacteria inhabiting host mucosal surfaces, NTHi has on its surface a diverse array of lipooligosaccharides (LOS) that influence host-bacterial interactions. In this study, we show that LOS containing sialic (N-acetyl-neuraminic) acid promotes biofilm formation by NTHi in vitro and bacterial persistence within the middle ear or lung in vivo. LOS from NTHi in biofilms was sialylated, as determined by comparison of electrophoretic mobilities and immunochemical reactivities before and after neuraminidase treatment. Biofilm formation was significantly reduced in media lacking sialic acid, and a siaB (CMP-sialic acid synthetase) mutant was deficient in biofilm formation in three different in vitro model systems. The persistence of an asialylated siaB mutant was attenuated in a gerbil middle ear infection model system, as well as in a rat pulmonary challenge model system. These data show that sialylated LOS glycoforms promote biofilm formation by NTHi and persistence in vivo.
Figures


References
-
- Alder, J. D., P. J. Ewing, A. M. Nilius, M. Mitten, A. Tovcimak, A. Oleksijew, K. Jarvis, L. Paige, and S. K. T. Tanaka. 1998. Dynamics of clarithromycin and azithromycin efficacies against experimental Haemophilus influenzae pulmonary infection. Antimicrob. Agents Chemother. 42:2385-2390. - PMC - PubMed
-
- Bakaletz, L. O., T. M. Hoepf, T. F. DeMaria, and D. J. Lim. 1988. The effect of antecedent influenza A virus infection on the adherence of Hemophilus influenzae to chinchilla tracheal epithelium. Am. J. Otolaryngol. 9:127-134. - PubMed
-
- Bakaletz, L. O., B.-J. Kennedy, L. A. Novotny, G. Duquesne, J. Cohen, and Y. Lobet. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect. Immun. 67:2746-2762. - PMC - PubMed
-
- Bauer, S. H., M. Mansson, D. W. Hood, J. C. Richards, E. R. Moxon, and E. K. Schweda. 2001. A rapid and sensitive procedure for determination of 5-N-acetyl neuraminic acid in lipopolysaccharides of Haemophilus influenzae: a survey of 24 non-typeable H. influenzae strains. Carbohydr. Res. 335:251-260. - PubMed
-
- Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 100:8898-8903. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

