Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface
- PMID: 14688088
- PMCID: PMC344006
- DOI: 10.1128/IAI.72.1.114-122.2004
Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface
Abstract
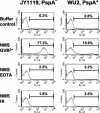
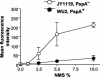
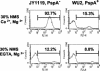
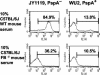
Streptococcus pneumoniae infection is a frequent cause of pneumonia, otitis media, meningitis, and septicemia. Pneumococcal surface protein A (PspA) is an important virulence factor on the pathogen surface, and it is known to interfere with complement activation. In this study, flow cytometry was used to study the effects of PspA and antibodies to PspA on the deposition of complement C3 on the surface of a capsular type 3 strain, WU2, and its PspA- mutant, JY1119. Using naive mouse serum as a complement source, measurable deposition of C3 was observed within 4 min on PspA- pneumococci, and the amount of surface-bound C3 accumulated rapidly as the amount of serum was increased. In contrast, very little C3 was deposited on the PspA+ strain. In nonimmune mouse serum, the classical pathway was the dominant activation pathway triggered by PspA- pneumococci. Accordingly, EGTA blocked almost all of the complement activation. Moreover, a significant amount of C3 was still deposited on the PspA- strain when serum from factor B-deficient mice was used. This deposition was not observed on the PspA+ pneumococci, indicating that PspA may inhibit complement deposition via the classical pathway. Furthermore, under the conditions we tested, PspA also inhibited C3 deposition when the classical pathway was initiated by antibodies to capsular polysaccharide. Antibodies to PspA could overcome the anticomplementary effect of PspA, allowing for increased complement activation and C3 deposition onto PspA+ bacteria.
Figures




Similar articles
-
Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae.Infect Immun. 2003 Jan;71(1):75-85. doi: 10.1128/IAI.71.1.75-85.2003. Infect Immun. 2003. PMID: 12496151 Free PMC article.
-
Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae.Infect Immun. 1999 Sep;67(9):4720-4. doi: 10.1128/IAI.67.9.4720-4724.1999. Infect Immun. 1999. PMID: 10456922 Free PMC article.
-
The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection.J Immunol. 2004 Dec 15;173(12):7506-12. doi: 10.4049/jimmunol.173.12.7506. J Immunol. 2004. PMID: 15585877
-
Systemic and mucosal protective immunity to pneumococcal surface protein A.Ann N Y Acad Sci. 1996 Oct 25;797:118-26. doi: 10.1111/j.1749-6632.1996.tb52954.x. Ann N Y Acad Sci. 1996. PMID: 8993356 Review.
-
A Jack of All Trades: The Role of Pneumococcal Surface Protein A in the Pathogenesis of Streptococcus pneumoniae.Front Cell Infect Microbiol. 2022 Feb 2;12:826264. doi: 10.3389/fcimb.2022.826264. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35186799 Free PMC article. Review.
Cited by
-
The absence of PspA or presence of antibody to PspA facilitates the complement-dependent phagocytosis of pneumococci in vitro.Clin Vaccine Immunol. 2012 Oct;19(10):1574-82. doi: 10.1128/CVI.00393-12. Epub 2012 Aug 1. Clin Vaccine Immunol. 2012. PMID: 22855389 Free PMC article.
-
Pneumococal Surface Protein A (PspA) Regulates Programmed Death Ligand 1 Expression on Dendritic Cells in a Toll-Like Receptor 2 and Calcium Dependent Manner.PLoS One. 2015 Jul 27;10(7):e0133601. doi: 10.1371/journal.pone.0133601. eCollection 2015. PLoS One. 2015. PMID: 26214513 Free PMC article.
-
Effect of apolactoferrin on experimental pneumococcal otitis media.Arch Otolaryngol Head Neck Surg. 2010 Nov;136(11):1127-31. doi: 10.1001/archoto.2010.192. Arch Otolaryngol Head Neck Surg. 2010. PMID: 21079169 Free PMC article.
-
Rationale and prospects for novel pneumococcal vaccines.Hum Vaccin Immunother. 2016;12(2):383-92. doi: 10.1080/21645515.2015.1087625. Hum Vaccin Immunother. 2016. PMID: 26535755 Free PMC article. Review.
-
Broadly Reactive Human Monoclonal Antibodies Targeting the Pneumococcal Histidine Triad Protein Protect against Fatal Pneumococcal Infection.Infect Immun. 2021 Apr 16;89(5):e00747-20. doi: 10.1128/IAI.00747-20. Print 2021 Apr 16. Infect Immun. 2021. PMID: 33649050 Free PMC article.
References
-
- Abeyta, M. 1999. Pneumococcal surface protein A and capsular polysaccharide in virulence of Streptococcus pneumoniae. Ph D. thesis. University of Alabama at Birmingham, Birmingham.
-
- Angel, C. S., M. Ruzek, and M. K. Hostetter. 1994. Degradation of C3 by Streptococcus pneumoniae. J. Infect. Dis. 170:600-608. - PubMed
-
- Briles, D. E., J. L. Claflin, K. Schroer, and C. Forman. 1981. Mouse IgG3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature 294:88-90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

