Immunogenicity and efficacy of Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan peptide mimotope-protein conjugates in human immunoglobulin transgenic mice
- PMID: 14688097
- PMCID: PMC343982
- DOI: 10.1128/IAI.72.1.196-208.2004
Immunogenicity and efficacy of Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan peptide mimotope-protein conjugates in human immunoglobulin transgenic mice
Abstract
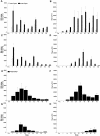
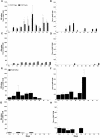
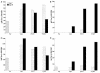
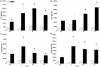
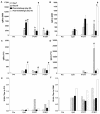
Peptide mimotopes of capsular polysaccharides have been proposed as antigens for vaccines against encapsulated pathogens. In this study, we determined the antibody response to and efficacy of P13, a peptide mimetic of the Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan (GXM), in mice that produce human antibodies. P13 was conjugated to tetanus toxoid (TT) or diphtheria toxoid (DT) and administered subcutaneously in Alhydrogel with or without CpG to mice transgenic for human immunoglobulin loci (XenoMouse mice) and expressing either immunoglobulin G2 (IgG2) (G2 mice) or IgG4 (G4 mice). Mice were vaccinated and revaccinated two or three times. The serum antibody responses of the mice to GXM and P13 and antibody idiotype expression were analyzed by an enzyme-linked immunosorbent assay. The results showed that both P13-TT and P13-DT were antigenic, inducing a mimetic response to P13 in both G2 and G4 mice, and immunogenic, inducing a mimotope response including VH3 (idiotype)-positive antibodies to GXM in G2 but not G4 mice. CpG led to higher titers of IgG to P13 and GXM in P13-TT-vaccinated G2 mice. C. neoformans challenge of P13-protein conjugate-vaccinated and control G2 mice induced anamnestic IgG- and VH3-positive responses to GXM and was associated with a significantly decreased risk of death and a prolongation of survival in P13-DT-vaccinated mice compared to phosphate-buffered saline-treated or protein carrier-vaccinated mice. These findings reveal that P13 elicited a human antibody response with VH3 expression in human immunoglobulin transgenic mice that has been observed for human antibodies to GXM and support the concept that peptide mimotope-based vaccines may hold promise for the treatment of C. neoformans infections.
Figures
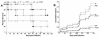
Similar articles
-
Therapeutic efficacy of a conjugate vaccine containing a peptide mimotope of cryptococcal capsular polysaccharide glucuronoxylomannan.Clin Vaccine Immunol. 2008 Aug;15(8):1176-87. doi: 10.1128/CVI.00130-08. Epub 2008 Jun 4. Clin Vaccine Immunol. 2008. PMID: 18524882 Free PMC article.
-
Efficacy of immune sera from human immunoglobulin transgenic mice immunized with a peptide mimotope of Cryptococcus neoformans glucuronoxylomannan.Vaccine. 2004 Sep 28;22(29-30):4062-8. doi: 10.1016/j.vaccine.2004.03.060. Vaccine. 2004. PMID: 15364457
-
A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection.J Immunol. 2001 Jan 15;166(2):1087-96. doi: 10.4049/jimmunol.166.2.1087. J Immunol. 2001. PMID: 11145689
-
Polysaccharides, mimotopes and vaccines for fungal and encapsulated pathogens.Trends Microbiol. 2001 Sep;9(9):445-51. doi: 10.1016/s0966-842x(01)02134-5. Trends Microbiol. 2001. PMID: 11553457 Review.
-
Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model.Vaccine. 1996 Jun;14(9):841-4. doi: 10.1016/0264-410x(95)00256-z. Vaccine. 1996. PMID: 8843625 Review.
Cited by
-
Application of anti-fungal vaccines as a tool against emerging anti-fungal resistance.Front Fungal Biol. 2023 Aug 21;4:1241539. doi: 10.3389/ffunb.2023.1241539. eCollection 2023. Front Fungal Biol. 2023. PMID: 37746132 Free PMC article. Review.
-
Molecular characterization of the early B cell response to pulmonary Cryptococcus neoformans infection.J Immunol. 2012 Dec 15;189(12):5820-30. doi: 10.4049/jimmunol.1201514. Epub 2012 Nov 21. J Immunol. 2012. PMID: 23175699 Free PMC article.
-
Carbohydrate moieties as vaccine candidates.Clin Infect Dis. 2005 Sep 1;41(5):705-12. doi: 10.1086/432582. Epub 2005 Jul 25. Clin Infect Dis. 2005. PMID: 16080094 Free PMC article. Review.
-
Fungal vaccines and adjuvants: a tool to reveal the interaction between host and fungi.Arch Microbiol. 2024 Jun 8;206(7):293. doi: 10.1007/s00203-024-04010-7. Arch Microbiol. 2024. PMID: 38850421 Review.
-
Therapeutic efficacy of a conjugate vaccine containing a peptide mimotope of cryptococcal capsular polysaccharide glucuronoxylomannan.Clin Vaccine Immunol. 2008 Aug;15(8):1176-87. doi: 10.1128/CVI.00130-08. Epub 2008 Jun 4. Clin Vaccine Immunol. 2008. PMID: 18524882 Free PMC article.
References
-
- Aalberse, R. C., J. Schuurman, and R. Van Ree. 1999. The apparent monovalency of human IgG4 is due to bispecificity. Int. Arch. Allergy Immunol. 118:187-189. - PubMed
-
- Abadi, J., J. Friedman, R. Jefferis, R. A. Mageed, and L. Pirofski. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J. Infect. Dis. 178:707-716. - PubMed
-
- Abadi, J., and L. Pirofski. 1999. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without HIV infection. J. Infect. Dis. 180:915-919. - PubMed
-
- Aberg, J. A., R. W. Price, D. M. Heeren, and B. Bredt. 2002. A pilot study of the discontinuation of antifungal therapy for disseminated cryptococcal disease in patients with acquired immunodeficiency syndrome, following immunologic response to antiretroviral therapy. J. Infect. Dis. 185:1179-1182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

