Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes
- PMID: 14688100
- PMCID: PMC344007
- DOI: 10.1128/IAI.72.1.229-237.2004
Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes
Abstract
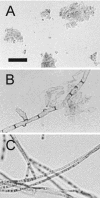
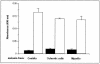
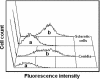
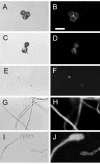
Fonsecaea pedrosoi is a fungal pathogen that produces melanin. The functions of melanin and its possible influence in the protective immunological response during infection by F. pedrosoi are not known. In this work, treatment of F. pedrosoi mycelia with proteases and glycosidases followed by a denaturing agent and hot concentrated acid left a black residue. Scanning electron microscopy demonstrated that this processed melanized residue resembled very closely the intact mycelium in shape and size. Melanin particles were also isolated from culture fluids of conidia or sclerotic forms of F. pedrosoi. Secreted melanins were reactive with sera from infected human patients, suggesting that F. pedrosoi synthesizes melanin in vivo. The antibodies against melanin were purified from patients' sera and analyzed by indirect immunofluorescence. They reacted with sclerotic cells from patients' lesions as well as with sclerotic bodies cultivated in vitro, conidia, mycelia, and digested residues. Treatment of F. pedrosoi with purified antibodies against melanin inhibited fungal growth in vitro. The interaction of F. pedrosoi with phagocytes in the presence of melanin resulted in higher levels of fungal internalization and destruction by host cells, which was accompanied by greater degrees of oxidative burst. Taken together, these results indicate that melanin from F. pedrosoi is an immunologically active fungal structure that activates humoral and cellular responses that could help the control of chromoblastomycosis by host defenses.
Figures




References
-
- Alviano, C. S., S. R. Farbiarz, W. De Souza, J. Angluster, and L. R. Travassos. 1991. Characterization of Fonsecaea pedrosoi melanin. J. Gen. Microbiol. 137:837-844. - PubMed
-
- Alviano, C. S., L. R. Travassos, J. Angluster, and W. De Souza. 1992. Effect of environmental factors on Fonsecaea pedrosoi morphogenesis with emphasis on sclerotic cells induced by propranolol. Mycopathologia 119:17-23. - PubMed
-
- Blinova, M. I., N. M. Yudintseva, N. V. Kalmykova, E. V. Kuzminykh, N. A. Yurlova, O. A. Ovchinnikova, and I. L. Potokin. 2003. Effect of melanins from black yeast fungi on proliferation and differentiation of cultivated human keratinocytes and fibroblasts. Cell. Biol. Int. 27:135-146. - PubMed
-
- Butterfield, W., and S. C. Jong. 1987. Effect of carbon source on conidiogenesis in Fonsecaea dermatitidis, agent of chromomycosis. Mycopathologia 58:59-62. - PubMed
-
- D'Acquisto, F., R. Carnuccio, M. D'Ischia, and G. Misuraca. 1995. 5,6-Dihydroxyindole-2-carboxylic acid, a diffusible melanin precursor, is a potent stimulator of lipopolysaccharide-induced production of nitric oxide by J774 macrophages. Life Sci. 57:401-406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

