Listeriosis in the pregnant guinea pig: a model of vertical transmission
- PMID: 14688130
- PMCID: PMC343973
- DOI: 10.1128/IAI.72.1.489-497.2004
Listeriosis in the pregnant guinea pig: a model of vertical transmission
Abstract
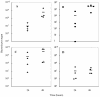
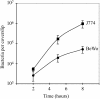
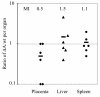
Feto-placental infections represent a major cause of pregnancy complications, and yet the underlying molecular and cellular mechanisms of vertical transmission are poorly understood. Listeria monocytogenes, a facultative intracellular pathogen, is one of a group of pathogens that are known to cause feto-placental infections in humans and other mammals. The purpose of this study was to evaluate possible mechanisms of vertical transmission of L. monocytogenes. Humans and guinea pigs have a hemochorial placenta, where a single layer of fetally derived trophoblasts separates maternal from fetal circulation. We characterized L. monocytogenes infection of the feto-placental unit in a pregnant guinea pig model and in primary human trophoblasts and trophoblast-derived cell lines. The clinical manifestations of listeriosis in the pregnant guinea pigs and the tropism of L. monocytogenes to the guinea pig placenta resembled those in humans. Trophoblast cell culture systems were permissive for listerial growth and cell-to-cell spread and revealed that L. monocytogenes deficient in internalin A, a virulence factor that mediates invasion of nonphagocytic cells, was 100-fold defective in invasion. However, crossing of the feto-placental barrier in the guinea pig model was independent of internalin A, suggesting a negligible role for internalin-mediated direct invasion of trophoblasts in vivo. Further understanding of vertical transmission of L. monocytogenes will help in designing more effective means of treatment and disease prevention.
Figures




References
-
- Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. - PubMed
-
- Braun, L., and P. Cossart. 2000. Interactions between Listeria monocytogenes and host mammalian cells. Microbes Infect. 2:803-811. - PubMed
-
- Cabanes, D., P. Dehoux, O. Dussurget, L. Frangeul, and P. Cossart. 2002. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 10:238-245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

