Shifting foci of hematopoiesis during reconstitution from single stem cells
- PMID: 14688412
- PMCID: PMC314166
- DOI: 10.1073/pnas.2637010100
Shifting foci of hematopoiesis during reconstitution from single stem cells
Abstract
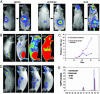
To reveal the early events and dynamics of hematopoietic reconstitution in living animals in real-time, we used bioluminescence imaging to monitor engraftment from single luciferase-labeled hematopoietic stem cells (HSC) in irradiated recipients. Transplanted HSC generated discrete foci in the spleen and bone marrow (BM), at a frequency that correlated with BM compartment size. Initially detected foci could expand locally, seed other sites in BM or spleen, and/or recede with different kinetics. These studies reveal dynamic and variable patterns of engraftment from highly purified HSC and indicate that the final overall contribution of individual HSC to hematopoietic chimerism does not depend on the specific site of initial engraftment and expansion.
Figures



References
-
- Spangrude, G. J., Heimfeld, S. & Weissman, I. L. (1988) Science 241, 58-62. - PubMed
-
- Morrison, S. J. & Weissman, I. L. (1994) Immunity 1, 661-673. - PubMed
-
- Hardy, C. L. (1995) Am. J. Med. Sci. 309, 260-266. - PubMed
-
- Hendrikx, P. J., Martens, C. M., Hagenbeek, A., Keij, J. F. & Visser, J. W. (1996) Exp. Hematol. (Charlottesville, Va) 24, 129-140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

