Histone H2A/H2B dimer exchange by ATP-dependent chromatin remodeling activities
- PMID: 14690611
- PMCID: PMC3428624
- DOI: 10.1016/s1097-2765(03)00499-4
Histone H2A/H2B dimer exchange by ATP-dependent chromatin remodeling activities
Abstract
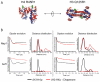
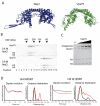
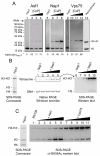
ATP-dependent chromatin remodeling activities function to manipulate chromatin structure during gene regulation. One of the ways in which they do this is by altering the positions of nucleosomes along DNA. Here we provide support for the ability of these complexes to move nucleosomes into positions in which DNA is unraveled from one edge. This is expected to result in the loss of histone-DNA contacts that are important for retention of one H2A/H2B dimer within the nucleosome. Consistent with this we find that several chromatin remodeling complexes are capable of catalyzing the exchange of H2A/H2B dimers between chromatin fragments in an ATP-dependent reaction. This provides eukaryotes with additional means by which they may manipulate chromatin structure.
Figures
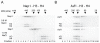

References
-
- Annunziato AT. Split decision: What happens to nucleosomes during DNA replication? Journal of Biological Chemistry. 2005;280:12065–12068. - PubMed
-
- Baxevanis AD, Godfrey JE, Moudrianakis EN. Associative behavior of the histone (H3-H4)2 Tetramer: Dependence on ionic environment. Biochemistry. 1991;30:8817–8823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

