Radiative decay engineering 3. Surface plasmon-coupled directional emission
- PMID: 14690679
- PMCID: PMC2737403
- DOI: 10.1016/j.ab.2003.09.039
Radiative decay engineering 3. Surface plasmon-coupled directional emission
Abstract
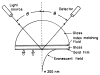
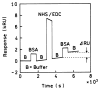
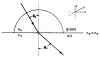
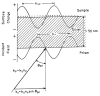
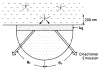
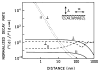
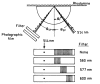
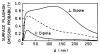
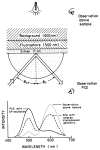
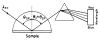
A new method of fluorescence detection that promises to increase sensitivity by 20- to 1000-fold is described. This method will also decrease the contribution of sample autofluorescence to the detected signal. The method depends on the coupling of excited fluorophores with the surface plasmon resonance present in thin metal films, typically silver and gold. The phenomenon of surface plasmon-coupled emission (SPCE) occurs for fluorophores 20-250 nm from the metal surface, allowing detection of fluorophores over substantial distances beyond the metal-sample interface. SPCE depends on interactions of the excited fluorophore with the metal surface. This interaction is independent of the mode of excitation; that is, it does not require evanescent wave or surface-plasmon excitation. In a sense, SPCE is the inverse process of the surface plasmon resonance absorption of thin metal films. Importantly, SPCE occurs over a narrow angular distribution, converting normally isotropic emission into easily collected directional emission. Up to 50% of the emission from unoriented samples can be collected, much larger than typical fluorescence collection efficiencies near 1% or less. SPCE is due only to fluorophores near the metal surface and may be regarded as emission from the induced surface plasmons. Autofluorescence from more distal parts of the sample is decreased due to decreased coupling. SPCE is highly polarized and autofluorescence can be further decreased by collecting only the polarized component or only the light propagating with the appropriate angle. Examples showing how simple optical configurations can be used in diagnostics, sensing, or biotechnology applications are presented. Surface plasmon-coupled emission is likely to find widespread applications throughout the biosciences.
Figures





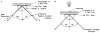
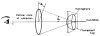






References
-
- Gosling JP. A decade of development in immunoassay methodology. Cell. 1990;36:1408–1427. - PubMed
-
- Walker NJ. A technique whose time has come. Science. 2002;296:557–559. - PubMed
-
- White JC, Stryer L. Photostability studies of phycobiliprotein fluorescent labels. Anal Biochem. 1987;161:442–452. - PubMed
-
- Kronick MN. The use of phycobiliproteins as fluorescent labels in immunoassays. J Immunol Methods. 1986;92:1–13. - PubMed
-
- Daehne S, Resch-Genger U, Wolfbeis OS. Near-Infrared Dyes for High Technology Applications. Kluwer Academic Publishers; New York: 1998. p. 458.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

