Radiative decay engineering 4. Experimental studies of surface plasmon-coupled directional emission
- PMID: 14690680
- PMCID: PMC2740992
- DOI: 10.1016/j.ab.2003.09.036
Radiative decay engineering 4. Experimental studies of surface plasmon-coupled directional emission
Abstract
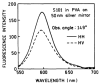
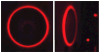
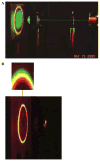
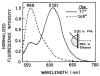
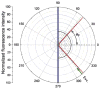
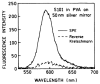
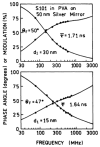
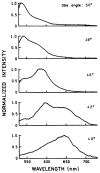
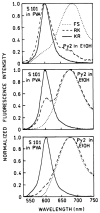
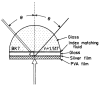
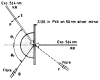
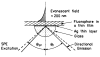
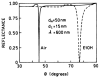
Fluorescence is typically isotropic in space and collected with low efficiency. In this paper we describe surface plasmon-coupled emission (SPCE), which displays unique optical properties and can be collected with an efficiency near 50%. SPCE occurs for fluorophores within about 200 nm of a thin metallic film, in our case a 50-nm-thick silver film on a glass substrate. We show that fluorophore proximity to this film converts the normally isotropic emission into highly directional emission through the glass substrate at a well-defined angle from the normal axis. Depending on the thickness of the polyvinyl alcohol (PVA) film on the silver, the coupling efficiency of sulforhodamine 101 in PVA ranged from 30 to 49%. Directional SPCE was observed whether the fluorophore was excited directly or by the evanescent field due to the surface plasmon resonance. The emission is always polarized perpendicular to the plane of incidence, irrespective of the polarization of the incident light. The lifetimes are not substantially changed, indicating a mechanism somewhat different from that observed previously for the effects of silver particles on fluorophores. Remarkably, the directional emission shows intrinsic spectral resolution because the coupling angles depend on wavelength. The distances over which SPCE occurs, 10 to 200 nm, are useful because a large number of fluorophores can be localized within this volume. The emission of more distant fluorophores does not couple into the glass, allowing background suppression from biological samples. SPCE can be expected to become rapidly useful in a variety of analytical and medical sensing applications.
Figures

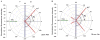







References
-
- Diamandis EP. Immunoassays with time-resolved fluorescence spectroscopy: principles and applications. Clin Biochem. 1988;21:139–150. - PubMed
-
- Lövgren T, Pettersson K. Time-resolved fluoroimmunoassay, advantages and limitations. In: Van Dyke K, Van Dyke R, editors. Luminescence Immunoassay and Molecular Applications. CRC Press; New York: 1990. pp. 234–250.
-
- White JC, Stryer L. Photostability studies of phycobiliprotein fluorescent labels. Anal Biochem. 1987;161:442–452. - PubMed
-
- Kronick MN. The use of phycobiliproteins as fluorescent labels in immunoassays. J Immunol Methods. 1986;92:1–13. - PubMed
-
- Ambrose WP, Goodwin PM, Jett JH, Van Orden A, Wemer JH, Keller RA. Single molecule fluorescence spectroscopy at ambient temperature. Chem Rev. 1999;99:2929–2956. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

