Two different proteins that compete for binding to thrombin have opposite kinetic and thermodynamic profiles
- PMID: 14691232
- PMCID: PMC2286536
- DOI: 10.1110/ps.03120604
Two different proteins that compete for binding to thrombin have opposite kinetic and thermodynamic profiles
Abstract
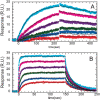
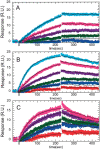
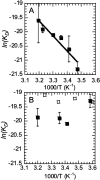
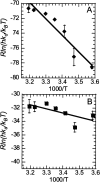
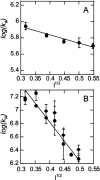
Thrombin binds thrombomodulin (TM) at anion binding exosite 1, an allosteric site far from the thrombin active site. A monoclonal antibody (mAb) has been isolated that competes with TM for binding to thrombin. Complete binding kinetic and thermodynamic profiles for these two protein-protein interactions have been generated. Binding kinetics were measured by Biacore. Although both interactions have similar K(D)s, TM binding is rapid and reversible while binding of the mAb is slow and nearly irreversible. The enthalpic contribution to the DeltaG(bind) was measured by isothermal titration calorimetry and van't Hoff analysis. The contribution to the DeltaG(bind) from electrostatic steering was assessed from the dependence of the k(a) on ionic strength. Release of solvent H(2)O molecules from the interface was assessed by monitoring the decrease in amide solvent accessibility at the interface upon protein-protein binding. The mAb binding is enthalpy driven and has a slow k(d). TM binding appears to be entropy driven and has a fast k(a). The favorable entropy of the thrombin-TM interaction seems to be derived from electrostatic steering and a contribution from solvent release. The two interactions have remarkably different thermodynamic driving forces for competing reactions. The possibility that optimization of binding kinetics for a particular function may be reflected in different thermodynamic driving forces is discussed.
Figures


References
-
- Baerga-Ortiz, A. 2002. “Energetics of the thrombin:thrombomodulin interaction.” Ph.D. thesis, University of California, San Diego.
-
- Baerga-Ortiz, A., Rezaie, A.R., and Komives, E.A. 2000. Electrostatic dependence of the thrombin–thrombomodulin interaction. J. Mol. Biol. 296 651–658. - PubMed
-
- Baker, B.M. and Murphy, K.P. 1997. Dissecting the energetics of a protein–protein interaction: The binding of ovomucoid third domain to elastase. J. Mol. Biol. 268 557–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

