Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors
- PMID: 14691258
- PMCID: PMC314193
- DOI: 10.1073/pnas.0304699101
Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors
Abstract
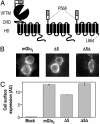
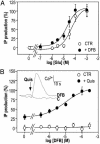
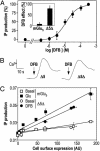
Although agonists bind directly in the heptahelical domain (HD) of most class-I rhodopsin-like G protein coupled receptors (GPCRs), class-III agonists bind in the extracellular domain of their receptors. Indeed, the latter possess a large extracellular domain composed of a cysteine-rich domain and a Venus flytrap module. Both the low sequence homology and the structural organization of class-III GPCRs raised the question of whether or not the HD of these receptors functions the same way as rhodopsin-like GPCRs. Here, we show that the HD of metabotropic glutamate receptor 5 (mGlu(5)) displays the same agonist-independent constitutive activity as the wild-type receptor. Moreover, we show that the noncompetitive antagonist MPEP [2-methyl-6-(phenylethynyl)-pyridine hydrochloride] and the positive allosteric modulator DFB (3,3'-difluorobenzaldazine) act as inverse agonist and full agonist, respectively, on the mGlu(5) HD in the absence of the extracellular domain. This finding illustrates that, like rhodopsin-like receptors, the HD of mGluRs can constitutively couple to G proteins and be negatively and positively regulated by ligands. These data show that the HD of mGluRs behave like any other class-I GPCRs in terms of G protein coupling and regulation by various types of ligands.
Figures



References
-
- Bockaert, J., Claeysen, S., Becamel, C., Pinloche, S. & Dumuis, A. (2002) Int. Rev. Cytol. 212, 63-132. - PubMed
-
- Fredriksson, R., Lagerstrom, M. C., Lundin, L. G. & Schioth, H. B. (2003) Mol. Pharmacol. 63, 1256-1272. - PubMed
-
- Lefkowitz, R. J., Cotecchia, S., Samama, P. & Costa, T. (1993) Trends Pharmacol. Sci. 14, 303-307. - PubMed
-
- Leff, P. (1995) Trends Pharmacol. Sci. 16, 89-97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

