Architecture and selectivity in aquaporins: 2.5 a X-ray structure of aquaporin Z
- PMID: 14691544
- PMCID: PMC300682
- DOI: 10.1371/journal.pbio.0000072
Architecture and selectivity in aquaporins: 2.5 a X-ray structure of aquaporin Z
Abstract
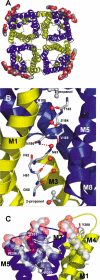
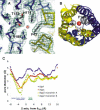
Aquaporins are a family of water and small molecule channels found in organisms ranging from bacteria to animals. One of these channels, the E. coli protein aquaporin Z (AqpZ), has been shown to selectively conduct only water at high rates. We have expressed, purified, crystallized, and solved the X-ray structure of AqpZ. The 2.5 A resolution structure of AqpZ suggests aquaporin selectivity results both from a steric mechanism due to pore size and from specific amino acid substitutions that regulate the preference for a hydrophobic or hydrophilic substrate. This structure provides direct evidence on the molecular mechanisms of specificity between water and glycerol in this family of channels from a single species. It is to our knowledge the first atomic resolution structure of a recombinant aquaporin and so provides a platform for combined genetic, mutational, functional, and structural determinations of the mechanisms of aquaporins and, more generally, the assembly of multimeric membrane proteins.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures


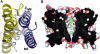
References
-
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. - PubMed
-
- Bernal JD, Fowler RH. A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J Chem Phys. 1933;1:515–548.
-
- Borgnia MJ, Kozono D, Calamita G, Maloney PC, Agre P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J Mol Biol. 1999a;291:1169–1179. - PubMed
-
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999b;68:425–458. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

