In vivo pharmacodynamic activity of daptomycin
- PMID: 14693519
- PMCID: PMC310158
- DOI: 10.1128/AAC.48.1.63-68.2004
In vivo pharmacodynamic activity of daptomycin
Abstract
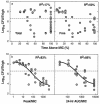
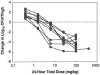
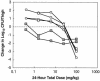
Daptomycin is a lipopeptide antibiotic with activity against a wide range of gram-positive bacteria. We used the neutropenic murine thigh model to characterize the pharmacodynamics of daptomycin. ICR/Swiss mice were rendered neutropenic with cyclophosphamide; and the thigh muscles of the mice were infected with strains of Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecium. Animals were treated by subcutaneous injection of daptomycin at doses of 0.20 to 400 mg/kg of body weight/day divided into one, two, four, or eight doses over 24 h. Daptomycin exhibited linear pharmacokinetics, with an area under the concentration-time curve (AUC) from time zero to infinity/dose of 9.4 and a half-life of 0.9 to 1.4 h. The level of protein binding was 90%. Free daptomycin exhibited concentration-dependent killing and produced in vivo postantibiotic effects (PAEs) of 4.8 to 10.8 h. Nonlinear regression analysis was used to determine which pharmacokinetic (PK) or pharmacodynamic (PD) parameter was important for efficacy by using free drug concentrations. The peak concentration/MIC (peak/MIC) ratio and 24-h AUC/MIC ratio were the PK and PD parameters that best correlated with in vivo efficacy (R(2) = 83 to 87% for peak/MIC and R(2) = 86% for the AUC/MIC ratio, whereas R(2) = 47 to 50% for the time that the concentration was greater than the MIC) against standard strains of S. aureus and S. pneumoniae. The peak/MIC ratios required for a bacteriostatic effect ranged from 12 to 36 for S. pneumoniae, 59 to 94 for S. aureus, and 0.14 to 0.25 for E. faecium. The AUC/MIC ratios needed for a bacteriostatic effect ranged from 75 to 237 for S. pneumoniae, 388 to 537 for S. aureus, and 0.94 to 1.67 for E. faecium. The free daptomycin concentrations needed to average from one to two times the MIC over 24 h to produce a bacteriostatic effect and two to four times the MIC over 24 h to produce greater than 99% killing. The long PAE and potent bactericidal activity make daptomycin an attractive option for the treatment of infections caused by gram-positive bacteria.
Figures



References
-
- Amsterdam, D., E. A. Gorzynski, T. R. Beam, and C. Rotstein. 1994. Susceptibility of bacteraemic isolates of gram-positive cocci to daptomycin and other antimicrobial agents. J. Antimicrob. Chemother. 33:1060-1064. - PubMed
-
- Andrew, J. H., M. C. Wale, L. J. Wale, and D. Greenwood. 1987. The effect of cultural conditions on the activity of LY146032 against staphylococci and streptococci. J. Antimicrob. Chemother. 20:213-221. - PubMed
-
- Anonymous. 1997. Minimum inhibitory concentration interpretive standards, M7-A4. Document 2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
-
- Anonymous. 1993. Nosocomial enterococci resistant to vancomycin—United States, 1989-1993. Morb. Mortal. Wkly. Rep. 42:597-599. - PubMed
-
- Appelbaum, P. C., S. K. Spangler, E. Crotty, and M. R. Jacobs. 1989. Susceptibility of penicillin-sensitive and -resistant strains of Streptococcus pneumoniae to new antimicrobial agents, including daptomycin, teicoplanin, cefpodoxime and quinolones. J. Antimicrob. Chemother. 23:509-516. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

