Sensitive enzyme immunoassay for measuring plasma and intracellular nevirapine levels in human immunodeficiency virus-infected patients
- PMID: 14693526
- PMCID: PMC310181
- DOI: 10.1128/AAC.48.1.104-109.2004
Sensitive enzyme immunoassay for measuring plasma and intracellular nevirapine levels in human immunodeficiency virus-infected patients
Abstract
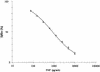
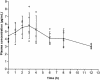
We have developed an enzyme immunoassay to measure nevirapine (NVP) in plasma and peripheral blood mononuclear cells. Anti-NVP polyclonal antibodies were raised in rabbits by using a synthetic NVP derivative coupled to keyhole limpet hemocyanin as the immunogen, and the enzyme tracer was prepared by chemically coupling the NVP derivative with acetylcholinesterase. These reagents were used to develop a sensitive competitive enzyme immunoassay performed in microtitration plates with a 100-pg ml(-1) limit of detection and thus approximately 100 times more sensitive than previously published techniques. The plasma assay was performed directly without extraction (in this case, a 500-pg ml(-1) limit of detection was observed) on a minimum of 30 micro l of plasma. This assay shows good precision and efficiency, since recovery from human plasma and cell extracts spiked with NVP ranged between 87 and 104%, with coefficients of variation of <10%. A pharmacokinetic analysis of plasma NVP was performed for seven patients infected with human immunodeficiency virus (HIV), and it gave results similar to published findings. Intracellular concentrations of NVP were measured in cultured human T-lymphoblastoid cells and peripheral blood mononuclear cells from HIV-infected patients. The results indicated a very low intracellular/extracellular concentration ratio (0.134), thus demonstrating the absence of intracellular drug accumulation. This is the first intracellular assay of a nonnucleoside reverse-transcriptase inhibitor, and this method could be useful in monitoring plasma and intracellular NVP levels in HIV-infected patients.
Figures

References
-
- Akeb, F., B. Ferrua, C. Creminon, C. Roptin, J. Grassi, M. C. Nevers, R. Guedj, R. Garraffo, and D. Duval. 2002. Quantification of plasma and intracellular levels of the HIV protease inhibitor ritonavir by competitive ELISA. J. Immunol. Methods 263:1-9. - PubMed
-
- Boffito, M., K. Sciole, R. Raiteri, S. Bonora, P. G. Hoggard, D. J. Back, and G. Di Perri. 2002. Alpha 1-acid glycoprotein levels in human immunodeficiency virus-infected subjects on antiretroviral regimens. Drug Metab. Dispos. 30:859-860. - PubMed
-
- Chaillou, S., J. Durant, R. Garraffo, E. Georgenthum, C. Roptin, B. Dunais, V. Mondain, P. M. Roger, and P. Dellamonica. 2002. Intracellular concentration of protease inhibitors in HIV-1-infected patients: correlation with MDR-1 gene expression and low dose of ritonavir. HIV Clin. Trials 3:493-501. - PubMed
-
- de Jong, M. D., S. Vella, A. Carr, C. A. Boucher, A. Imrie, M. French, J. Hoy, S. Sorice, S. Pauluzzi, F. Chiodo, G. J. Weverling, M. E. van der Ende, P. J. Frissen, H. M. Weigel, R. H. Kauffmann, J. M. Lange, R. Yoon, M. Moroni, E. Hoenderdos, G. Leitz, D. A. Cooper, D. Hall, and P. Reiss. 1997. High-dose nevirapine in previously untreated human immunodeficiency virus type 1-infected persons does not result in sustained suppression of viral replication. J. Infect. Dis. 175:966-970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

