Neutrality of the canonical NF-kappaB-dependent pathway for human and murine cytomegalovirus transcription and replication in vitro
- PMID: 14694106
- PMCID: PMC368812
- DOI: 10.1128/jvi.78.2.741-750.2004
Neutrality of the canonical NF-kappaB-dependent pathway for human and murine cytomegalovirus transcription and replication in vitro
Abstract
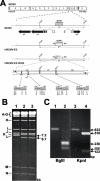
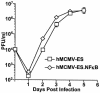
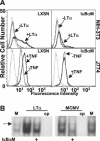
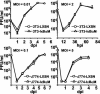
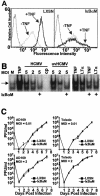
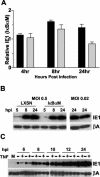
Cytomegalovirus (CMV) is known to rapidly induce activation of nuclear factor kappaB (NF-kappaB) after infection of fibroblast and macrophage cells. NF-kappaB response elements are present in the enhancer region of the CMV major immediate-early promoter (MIEP), and activity of the MIEP is strongly upregulated by NF-kappaB in transient-transfection assays. Here we investigate whether the NF-kappaB-dependent pathway is required for initiating or potentiating human and murine CMV replication in vitro. We show that expression of a dominant negative mutant of the inhibitor of NF-kappaB-alpha (IkappaBalphaM) does not alter the replication kinetics of human or mouse CMV in cultured cells. In addition, mouse embryo fibroblasts genetically deficient for p65/RelA actually showed elevated levels of MCMV replication. Mutation of all NF-kappaB response elements within the enhancer of the MIEP in a recombinant mouse CMV containing the human MIEP (hMCMV-ES), which we have previously shown to replicate in murine fibroblasts with kinetics equivalent to that of wild-type mouse CMV, did not negatively affect replication in fibroblasts. Taken together, these data show that, for CMV replication in cultured fibroblasts activation of the canonical NF-kappaB pathway and binding of NF-kappaB to the MIEP are dispensable, and in the case of p65 may even interfere, thus uncovering a previously unrecognized level of complexity in the host regulatory network governing MIE gene expression in the context of a viral infection.
Figures

Similar articles
-
The activator protein 1 binding motifs within the human cytomegalovirus major immediate-early enhancer are functionally redundant and act in a cooperative manner with the NF-{kappa}B sites during acute infection.J Virol. 2011 Feb;85(4):1732-46. doi: 10.1128/JVI.01713-10. Epub 2010 Nov 24. J Virol. 2011. PMID: 21106746 Free PMC article.
-
The Elk-1 and serum response factor binding sites in the major immediate-early promoter of human cytomegalovirus are required for efficient viral replication in quiescent cells and compensate for inactivation of the NF-kappaB sites in proliferating cells.J Virol. 2010 May;84(9):4481-93. doi: 10.1128/JVI.02141-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147408 Free PMC article.
-
Activation of the virus-induced IKK/NF-kappaB signalling axis is critical for the replication of human cytomegalovirus in quiescent cells.Cell Microbiol. 2007 Aug;9(8):2040-54. doi: 10.1111/j.1462-5822.2007.00936.x. Epub 2007 Apr 5. Cell Microbiol. 2007. PMID: 17419715
-
Bcl-3-regulated transcription from major immediate-early promoter of human cytomegalovirus in monocyte-derived macrophages.J Immunol. 2009 Jun 15;182(12):7784-94. doi: 10.4049/jimmunol.0803800. J Immunol. 2009. PMID: 19494302
-
The human cytomegalovirus major immediate-early enhancer determines the efficiency of immediate-early gene transcription and viral replication in permissive cells at low multiplicity of infection.J Virol. 2003 Mar;77(6):3602-14. doi: 10.1128/jvi.77.6.3602-3614.2003. J Virol. 2003. PMID: 12610136 Free PMC article.
Cited by
-
Role of the proximal enhancer of the major immediate-early promoter in human cytomegalovirus replication.J Virol. 2004 Dec;78(23):12788-99. doi: 10.1128/JVI.78.23.12788-12799.2004. J Virol. 2004. PMID: 15542631 Free PMC article.
-
Role of the cytomegalovirus major immediate early enhancer in acute infection and reactivation from latency.Med Microbiol Immunol. 2008 Jun;197(2):223-31. doi: 10.1007/s00430-007-0069-7. Epub 2007 Dec 19. Med Microbiol Immunol. 2008. PMID: 18097687 Review.
-
Reversible inhibition of murine cytomegalovirus replication by gamma interferon (IFN-γ) in primary macrophages involves a primed type I IFN-signaling subnetwork for full establishment of an immediate-early antiviral state.J Virol. 2011 Oct;85(19):10286-99. doi: 10.1128/JVI.00373-11. Epub 2011 Jul 20. J Virol. 2011. PMID: 21775459 Free PMC article.
-
DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA.Cell Host Microbe. 2012 Mar 15;11(3):290-7. doi: 10.1016/j.chom.2012.01.016. Cell Host Microbe. 2012. PMID: 22423968 Free PMC article.
-
New cell-signaling pathways for controlling cytomegalovirus replication.Am J Transplant. 2014 Jun;14(6):1249-58. doi: 10.1111/ajt.12725. Epub 2014 May 19. Am J Transplant. 2014. PMID: 24839861 Free PMC article. Review.
References
-
- Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782-784. - PubMed
-
- Benedict, C. A., T. A. Banks, L. Senderowicz, M. Ko, W. J. Britt, A. Angulo, P. Ghazal, and C. F. Ware. 2001. Lymphotoxins and cytomegalovirus cooperatively induce interferon-β, establishing host-virus détente. Immunity 15:617-626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

