Nuclear localization of avian polyomavirus structural protein VP1 is a prerequisite for the formation of virus-like particles
- PMID: 14694124
- PMCID: PMC368749
- DOI: 10.1128/jvi.78.2.930-937.2004
Nuclear localization of avian polyomavirus structural protein VP1 is a prerequisite for the formation of virus-like particles
Abstract
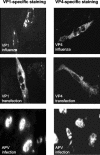
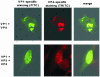
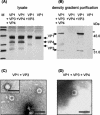
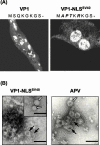
Virions of polyomaviruses consist of the major structural protein VP1, the minor structural proteins VP2 and VP3, and the viral genome associated with histones. An additional structural protein, VP4, is present in avian polyomavirus (APV) particles. As it had been reported that expression of APV VP1 in insect cells did not result in the formation of virus-like particles (VLP), the prerequisites for particle formation were analyzed. To this end, recombinant influenza viruses were created to (co)express the structural proteins of APV in chicken embryo cells, permissive for APV replication. VP1 expressed individually or coexpressed with VP4 did not result in VLP formation; both proteins (co)localized in the cytoplasm. Transport of VP1, or the VP1-VP4 complex, into the nucleus was facilitated by the coexpression of VP3 and resulted in the formation of VLP. Accordingly, a mutant APV VP1 carrying the N-terminal nuclear localization signal of simian virus 40 VP1 was transported to the nucleus and assembled into VLP. These results support a model of APV capsid assembly in which complexes of the structural proteins VP1, VP3 (or VP2), and VP4, formed within the cytoplasm, are transported to the nucleus using the nuclear localization signal of VP3 (or VP2); there, capsid formation is induced by the nuclear environment.
Figures



Similar articles
-
Avian polyomavirus major capsid protein VP1 interacts with the minor capsid proteins and is transported into the cell nucleus but does not assemble into capsid-like particles when expressed in the baculovirus system.Virus Res. 1999 Nov;64(2):173-85. doi: 10.1016/s0168-1702(99)00093-3. Virus Res. 1999. PMID: 10518713
-
Major and minor capsid proteins of human polyomavirus JC cooperatively accumulate to nuclear domain 10 for assembly into virions.J Virol. 2004 Sep;78(18):9890-903. doi: 10.1128/JVI.78.18.9890-9903.2004. J Virol. 2004. PMID: 15331723 Free PMC article.
-
Analysis of capsid formation of human polyomavirus JC (Tokyo-1 strain) by a eukaryotic expression system: splicing of late RNAs, translation and nuclear transport of major capsid protein VP1, and capsid assembly.J Virol. 2000 Feb;74(4):1840-53. doi: 10.1128/jvi.74.4.1840-1853.2000. J Virol. 2000. PMID: 10644357 Free PMC article.
-
[Development of intranuclear inclusions of human polyomavirus JC. Capsid proteins are assembled into virions at the PML nuclear bodies].Uirusu. 2006 Jun;56(1):17-25. doi: 10.2222/jsv.56.17. Uirusu. 2006. PMID: 17038808 Review. Japanese.
-
Recombinant virus like particles as drug delivery system.Curr Pharm Biotechnol. 2005 Feb;6(1):49-55. doi: 10.2174/1389201053167202. Curr Pharm Biotechnol. 2005. PMID: 15727555 Review.
Cited by
-
Polyomaviruses of birds: etiologic agents of inflammatory diseases in a tumor virus family.J Virol. 2007 Nov;81(21):11554-9. doi: 10.1128/JVI.01178-07. Epub 2007 Aug 22. J Virol. 2007. PMID: 17715213 Free PMC article. Review. No abstract available.
-
Characterization of two novel polyomaviruses of birds by using multiply primed rolling-circle amplification of their genomes.J Virol. 2006 Apr;80(7):3523-31. doi: 10.1128/JVI.80.7.3523-3531.2006. J Virol. 2006. PMID: 16537620 Free PMC article.
-
The structure of avian polyomavirus reveals variably sized capsids, non-conserved inter-capsomere interactions, and a possible location of the minor capsid protein VP4.Virology. 2011 Mar 1;411(1):142-52. doi: 10.1016/j.virol.2010.12.005. Epub 2011 Jan 15. Virology. 2011. PMID: 21239031 Free PMC article.
References
-
- An, K., S. A. Smiley, E. T. Gillock, W. M. Reeves, and R. A. Consigli. 1999. Avian polyomavirus major capsid protein VP1 interacts with the minor capsid proteins and is transported into the cell nucleus but does not assemble into capsid-like particles when expressed in the baculovirus system. Virus Res. 64:173-185. - PubMed
-
- Delos, S. E., L. Montross, R. B. Moreland, and R. L. Garcea. 1993. Expression of the polyomavirus VP2 and VP3 proteins in insect cells: coexpression with the major capsid protein VP1 alters VP2/VP3 subcellular localization. Virology 194:393-398. - PubMed
-
- Fattaey, A., L. Lenz, and R. A. Consigli. 1992. Production and characterization of monoclonal antibodies to budgerigar fledgling disease virus major capsid protein VP1. Avian Dis. 36:543-553. - PubMed
-
- Gedvilaite, A., C. Frommel, K. Sasnauskas, B. Micheel, M. Ozel, O. Behrsing, J. Staniulis, B. Jandrig, S. Scherneck, and R. Ulrich. 2000. Formation of immunogenic virus-like particles by inserting epitopes into surface-exposed regions of hamster polyomavirus major capsid protein. Virology 273:21-35. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

