Regulation of human immunodeficiency virus type 1 Env-mediated membrane fusion by viral protease activity
- PMID: 14694135
- PMCID: PMC368813
- DOI: 10.1128/jvi.78.2.1026-1031.2004
Regulation of human immunodeficiency virus type 1 Env-mediated membrane fusion by viral protease activity
Abstract
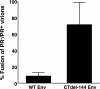
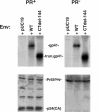
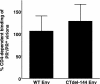
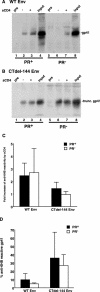
We and others have presented evidence for a direct interaction between the matrix (MA) domain of the human immunodeficiency virus type 1 (HIV-1) Gag protein and the cytoplasmic tail of the transmembrane envelope (Env) glycoprotein gp41. In addition, it has been postulated that the MA domain of Gag undergoes a conformational change following Gag processing, and the cytoplasmic tail of gp41 has been shown to modulate Env-mediated membrane fusion activity. Together, these results raise the possibility that the interaction between the gp41 cytoplasmic tail and MA is regulated by protease (PR)-mediated Gag processing, perhaps affecting Env function. To examine whether Gag processing affects Env-mediated fusion, we compared the ability of wild-type (WT) HIV-1 Env and a mutant lacking the gp41 cytoplasmic tail to induce fusion in the context of an active (PR(+)) or inactive (PR(-)) viral PR. We observed that PR(-) virions bearing WT Env displayed defects in cell-cell fusion. Impaired fusion did not appear to be due to differences in the levels of virion-associated Env, in CD4-dependent binding to target cells, or in the formation of the CD4-induced gp41 six-helix bundle. Interestingly, truncation of the gp41 cytoplasmic tail reversed the fusion defect. These results suggest that interactions between unprocessed Gag and the gp41 cytoplasmic tail suppress fusion.
Figures
References
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

