Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications
- PMID: 14694147
- PMCID: PMC1664875
- DOI: 10.1113/jphysiol.2003.055913
Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications
Abstract
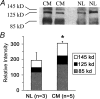
There is substantial evidence that oxidative stress participates in the pathophysiology of cardiovascular disease. Biochemical, molecular and pharmacological studies further implicate xanthine oxidoreductase (XOR) as a source of reactive oxygen species in the cardiovascular system. XOR is a member of the molybdoenzyme family and is best known for its catalytic role in purine degradation, metabolizing hypoxanthine and xanthine to uric acid with concomitant generation of superoxide. Gene expression of XOR is regulated by oxygen tension, cytokines and glucocorticoids. XOR requires molybdopterin, iron-sulphur centres, and FAD as cofactors and has two interconvertible forms, xanthine oxidase and xanthine dehydrogenase, which transfer electrons from xanthine to oxygen and NAD(+), respectively, yielding superoxide, hydrogen peroxide and NADH. Additionally, XOR can generate superoxide via NADH oxidase activity and can produce nitric oxide via nitrate and nitrite reductase activities. While a role for XOR beyond purine metabolism was first suggested in ischaemia-reperfusion injury, there is growing awareness that it also participates in endothelial dysfunction, hypertension and heart failure. Importantly, the XOR inhibitors allopurinol and oxypurinol attenuate dysfunction caused by XOR in these disease states. Attention to the broader range of XOR bioactivity in the cardiovascular system has prompted initiation of several randomised clinical outcome trials, particularly for congestive heart failure. Here we review XOR gene structure and regulation, protein structure, enzymology, tissue distribution and pathophysiological role in cardiovascular disease with an emphasis on heart failure.
Figures



References
-
- Abadeh S, Case PC, Harrison R. Purification of xanthine oxidase from human heart. Biochem Soc Trans. 1993;21:99S. - PubMed
-
- Abadeh S, Killacky J, Benboubetra M, Harrison R. Purification and partial characterization of xanthine oxidase from human milk. Biochim Biophys Acta. 1992;1117:25–32. - PubMed
-
- Alderman MH, Cohen H, Madhavan S, Kivlighn S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–150. - PubMed
-
- Amaya Y, Yamazaki K, Sato M, Noda K, Nishino T, Nishino T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J Biol Chem. 1990;265:14170–14175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

