Membrane potential stabilization in amphibian skeletal muscle fibres in hypertonic solutions
- PMID: 14694151
- PMCID: PMC1664835
- DOI: 10.1113/jphysiol.2003.058545
Membrane potential stabilization in amphibian skeletal muscle fibres in hypertonic solutions
Abstract
This study investigated membrane transport mechanisms influencing relative changes in cell volume (V) and resting membrane potential (E(m)) following osmotic challenge in amphibian skeletal muscle fibres. It demonstrated a stabilization of E(m) despite cell shrinkage, which was attributable to elevation of intracellular [Cl(-)] above electrochemical equilibrium through Na(+)-Cl(-) and Na(+)-K(+)-2Cl(-) cotransporter action following exposures to extracellular hypertonicity. Fibre volumes (V) determined by confocal microscope x z - scanning of cutaneous pectoris muscle fibres varied linearly with [1/extracellular osmolarity], showing insignificant volume corrections, in fibres studied in Cl(-)-free, normal and Na(+)-free Ringer solutions and in the presence of bumetanide, chlorothiazide and ouabain. The observed volume changes following increases in extracellular tonicity were compared with microelectrode measurements of steady-state resting potentials (E(m)). Fibres in isotonic Cl(-)-free, normal and Na(+)-free Ringer solutions showed similar E(m) values consistent with previously reported permeability ratios P(Na)/P(K)(0.03-0.05) and P(Cl)/P(K) ( approximately 2.0) and intracellular [Na(+)], [K(+)] and [Cl(-)]. Increased extracellular osmolarities produced hyperpolarizing shifts in E(m) in fibres studied in Cl(-)-free Ringer solution consistent with the Goldman-Hodgkin-Katz (GHK) equation. In contrast, fibres exposed to hypertonic Ringer solutions of normal ionic composition showed no such E(m) shifts, suggesting a Cl(-)-dependent stabilization of membrane potential. This stabilization of E(m) was abolished by withdrawing extracellular Na(+) or by the combined presence of the Na(+)-Cl(-) cotransporter (NCC) inhibitor chlorothiazide (10 microM) and the Na(+)-K(+)-2Cl(-) cotransporter (NKCC) inhibitor bumetanide (10 microM), or the Na(+)-K(+)-ATPase inhibitor ouabain (1 or 10 microM) during alterations in extracellular osmolarity. Application of such agents after such increases in tonicity only produced a hyperpolarization after a time delay, as expected for passive Cl(-) equilibration. These findings suggest a model that implicates the NCC and/or NKCC in fluxes that maintain [Cl(-)](i) above its electrochemical equilibrium. Such splinting of [Cl(-)](i) in combination with the high P(Cl)/P(K) of skeletal muscle stabilizes E(m) despite volume changes produced by extracellular hypertonicity, but at the expense of a cellular capacity for regulatory volume increases (RVIs). In situations where P(Cl)/P(K) is low, the same co-transporters would instead permit RVIs but at the expense of a capacity to stabilize E(m).
Figures












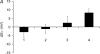
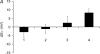
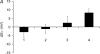


References
-
- Barry PH, Dulhunty AF. Slow potential changes in mammalian muscle fibers during prolonged hyperpolarization: transport number effects and chloride depletion. J Membr Biol. 1984;78:235–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

