Interplay of troponin- and Myosin-based pathways of calcium activation in skeletal and cardiac muscle: the use of W7 as an inhibitor of thin filament activation
- PMID: 14695278
- PMCID: PMC1303801
- DOI: 10.1016/S0006-3495(04)74112-0
Interplay of troponin- and Myosin-based pathways of calcium activation in skeletal and cardiac muscle: the use of W7 as an inhibitor of thin filament activation
Abstract
To investigate the interplay between the thin and thick filaments during calcium activation in striated muscle, we employed n-(6-aminohexyl) 5-chloro-1-napthalenesulfonamide (W7) as an inhibitor of troponin C and compared its effects with that of the myosin-specific inhibitor, 2,3-butanedione 2-monoxime (BDM). In both skeletal and cardiac fibers, W7 reversibly inhibited ATPase and tension over the full range of calcium activation between pCa 8.0 and 4.5, resulting in reduced calcium sensitivity and cooperativity of ATPase and tension activations. At maximal activation in skeletal fibers, the W7 concentrations for half-maximal inhibition (KI) were 70-80 micro M for ATPase and 20-30 micro M for tension, nearly >200-fold lower than BDM (20 mM and 5-8 mM, respectively). When W7 (50 microM) and BDM (20 mM) were combined in skeletal fibers, the ATPase and tension-pCa curves exhibited lower apparent cooperativity and maxima and higher calcium sensitivity than expected from two independent activation pathways, suggesting that the interplay between the thin and thick filaments varies with the level of activation. Significantly, the inhibition of W7 increased the ATPase/tension ratio during activation in both muscle types. W7 holds much promise as a potent and reversible inhibitor of thin filament-mediated calcium activation of skeletal and cardiac muscle contraction.
Figures
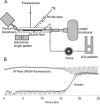


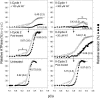


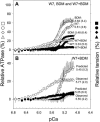


References
-
- Adhikari, B. B., and K. Wang. 2001. S100A1 modulates skeletal muscle contraction by desensitizing calcium activation of isometric tension, stiffness and ATPase. FEBS Lett. 497:95–98. - PubMed
-
- al-Khayat, H. A., N. Yagi, and J. M. Squire. 1995. Structural changes in actin-tropomyosin during muscle regulation: computer modeling of low-angle x-ray diffraction data. J. Mol. Biol. 252:611–632. - PubMed
-
- Allen, K., Y. Y. Xu, and W. G. Kerrick. 2000. Ca2+ measurements in skinned cardiac fibers: effects of Mg2+ on Ca2+ activation of force and fiber ATPase. J. Appl. Physiol. 88:180–185. - PubMed
-
- Bagshaw, C. R. 1993. Muscle Contraction. Chapman & Hal, London and New York.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

