Synaptic integration in electrically coupled neurons
- PMID: 14695308
- PMCID: PMC1303833
- DOI: 10.1016/S0006-3495(04)74142-9
Synaptic integration in electrically coupled neurons
Abstract
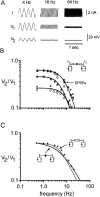
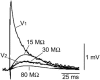
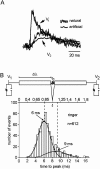
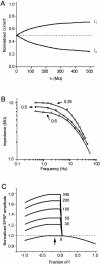
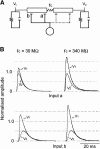
Interactions among chemical and electrical synapses regulate the patterns of electrical activity of vertebrate and invertebrate neurons. In this investigation we studied how electrical coupling influences the integration of excitatory postsynaptic potentials (EPSPs). Pairs of Retzius neurons of the leech are coupled by a nonrectifying electrical synapse by which chemically induced synaptic currents flow from one neuron to the other. Results from electrophysiology and modeling suggest that chemical synaptic inputs are located on the coupled neurites, at 7.5 microm from the electrical synapses. We also showed that the space constant of the coupled neurites was 100 microm, approximately twice their length, allowing the efficient spread of synaptic currents all along both coupled neurites. Based on this cytoarchitecture, our main finding was that the degree of electrical coupling modulates the amplitude of EPSPs in the driving neurite by regulating the leak of synaptic current to the coupled neurite, so that the amplitude of EPSPs in the driving neurite was proportional to the value of the coupling resistance. In contrast, synaptic currents arriving at the coupled neurite through the electrical synapse produced EPSPs of constant amplitude. This was because the coupling resistance value had inverse effects on the amount of current arriving and on the impedance of the neurite. We propose that by modulating the amplitude of EPSPs, electrical synapses could regulate the firing frequency of neurons.
Figures



References
-
- Bennett, M. V. L., E. Aljure, Y. Nakajima, and G. D. Pappas. 1963. Electrotonic junctions between teleost spinal neurons: electrophysiology and ultrastructure. Science. 141:262–264. - PubMed
-
- Bras, H., P. Gogan, and S. Tyc-Dumont. 1987. The dendrites of single brain-stem motoneurons intracellularly labeled with horseradish peroxidase in the cat. Morphological and electrical differences. Neuroscience. 22:947–970. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

