K-ras gene mutation enhances motility of immortalized airway cells and lung adenocarcinoma cells via Akt activation: possible contribution to non-invasive expansion of lung adenocarcinoma
- PMID: 14695323
- PMCID: PMC1602223
- DOI: 10.1016/S0002-9440(10)63100-8
K-ras gene mutation enhances motility of immortalized airway cells and lung adenocarcinoma cells via Akt activation: possible contribution to non-invasive expansion of lung adenocarcinoma
Abstract
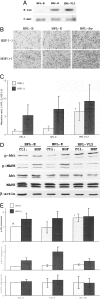
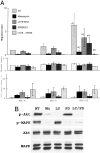
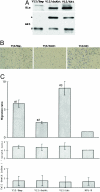
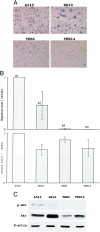
Point mutations of the K-ras gene, which are found in 10 to 30% of lung adenocarcinomas, are regarded as being an early event during the carcinogenesis. Autonomous vigorous motility of neoplastic cells, as well as growth and survival advantages, are considered to be necessary for cancer development and progression. The present study describes the contributions of the K-ras gene mutation and its downstream pathway via phosphatidylinositol 3-OH kinase (PI3K)-Akt to the cell motility in an immortalized human peripheral airway epithelial cell (HPL1D) and lung adenocarcinoma cells (A549, H820, TKB6, and TKB14). We have also evaluated the relationship between pathological events and the K-ras-Akt pathway using surgically resected lung tumors. The HPL1D cells transfected with the mutated K-ras gene (HPL-V12) showed a significant increase in cell motility compared to those transfected with empty vector (HPL-E) or wild-type K-ras gene (HPL-K). The enhanced motility in the HPL-V12 cells was markedly reduced by either treatment with inhibitors of ras, PI3K, and/or MEK, or by transfection with the dominant-negative mutant Akt (dnAkt). The lung adenocarcinoma cells bearing the K-ras gene mutation (A549 and H820) showed consistently higher levels of cell motilities than those without the mutation (TKB6 and TKB14), and the motility of A549 and H820 cells were significantly inhibited by dnAkt transfection. These results suggest that the K-ras gene mutation could enhance the motility of neoplastic cells through a pathway involving PI3K-Akt. Actually, among the surgically resected lung tumors, the adenocarcinomas with the K-ras gene mutation tended to show a higher frequency and intensity of immunoreactivity for phosphorylated Akt (p-ser473Akt) than those without the mutation, supporting the in vitro observation that the mutated K-ras can activate the PI3K-Akt pathway. Immunoreactivity for p-ser473Akt was also seen in the pre-malignant and early lesions at a frequency similar to that in the advanced lung adenocarcinomas,. No correlation was seen between p-ser473Akt immunoreactivity and lymphatic/organ metastasis or prognosis. These results taken together suggest that the K-ras-Akt pathway might facilitate the motility of neoplastic cells during the early period of carcinogenesis in lung adenocarcinomas, and may contribute to their non-invasive expansion along the alveolar septa, rather than invasion or metastasis.
Figures

References
-
- Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. - PubMed
-
- Bos JL, Verlaan-de Vries M, van der Eb AJ, Janssen JW, Delwel R, Lowenberg B, Colly LP. Mutations in N-ras predominate in acute myeloid leukemia. Blood. 1987;69:1237–1241. - PubMed
-
- Segrelle C, Ruiz S, Perez P, Murga C, Santos M, Budunova IV, Martinez J, Larcher F, Slaga TJ, Gutkind JS, Jorcan JL, Paramio JM. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. 2002;21:53–64. - PubMed
-
- Rodenhuis S, van de Wetering ML, Mooi WJ, Evers SG, van Zandwijk N, Bos JL. Mutational activation of the K-ras oncogene: a possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987;317:929–935. - PubMed
-
- Cooper CA, Carby FA, Bubb VJ, Lamb D, Kerr KM, Wyllie AH. The pattern of K-ras mutation in pulmonary adenocarcinoma defines a new pathway of tumor development in the human lung. J Pathol. 1997;181:401–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

