Entropic switch regulates myristate exposure in the HIV-1 matrix protein
- PMID: 14699046
- PMCID: PMC327179
- DOI: 10.1073/pnas.0305665101
Entropic switch regulates myristate exposure in the HIV-1 matrix protein
Abstract
The myristoylated matrix protein (myr-MA) of HIV functions as a regulator of intracellular localization, targeting the Gag precursor polyprotein to lipid rafts in the plasma membrane during virus assembly and dissociating from the membrane during infectivity for nuclear targeting of the preintegration complex. Membrane release is triggered by proteolytic cleavage of Gag, and it has, until now, been believed that proteolysis induces a conformational change in myr-MA that sequesters the myristyl group. NMR studies reported here reveal that myr-MA adopts myr-exposed [myr(e)] and -sequestered [myr(s)] states, as anticipated. Unexpectedly, the tertiary structures of the protein in both states are very similar, with the sequestered myristyl group occupying a cavity that requires only minor conformational adjustments for insertion. In addition, myristate exposure is coupled with trimerization, with the myristyl group sequestered in the monomer and exposed in the trimer (K(assoc) = 2.5 +/- 0.6 x 10(8) M(-2)). The equilibrium constant is shifted approximately 20-fold toward the trimeric, myristate-exposed species in a Gag-like construct that includes the capsid domain, indicating that exposure is enhanced by Gag subdomains that promote self-association. Our findings indicate that the HIV-1 myristyl switch is regulated not by mechanically induced conformational changes, as observed for other myristyl switches, but instead by entropic modulation of a preexisting equilibrium.
Figures
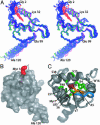

 [myr(e)-MA]3 equilibrium showing the experimentally determined NMR structure of myr(s)-MA and a proposed model for the [myr(e)-MA]3 trimer. The model was generated by superpositioning three identical copies of a representative NMR structure of myr-MA [generated by using only myr(–)-MA NMR restraints] onto the coordinates of the trimeric myr(–)-MA x-ray structure. The model is consistent with the NMR chemical shift changes observed for myr-MA at protein concentrations that favor self-association. Lysine residues that are important for efficient virus replication (67) and have been proposed to interact with the membrane surface (25) are shown in blue. The intermolecular myr–myr interactions may be disrupted upon binding to membranes.
[myr(e)-MA]3 equilibrium showing the experimentally determined NMR structure of myr(s)-MA and a proposed model for the [myr(e)-MA]3 trimer. The model was generated by superpositioning three identical copies of a representative NMR structure of myr-MA [generated by using only myr(–)-MA NMR restraints] onto the coordinates of the trimeric myr(–)-MA x-ray structure. The model is consistent with the NMR chemical shift changes observed for myr-MA at protein concentrations that favor self-association. Lysine residues that are important for efficient virus replication (67) and have been proposed to interact with the membrane surface (25) are shown in blue. The intermolecular myr–myr interactions may be disrupted upon binding to membranes.
Comment in
-
A myristoyl switch regulates membrane binding of HIV-1 Gag.Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):417-8. doi: 10.1073/pnas.0308043101. Epub 2004 Jan 5. Proc Natl Acad Sci U S A. 2004. PMID: 14707265 Free PMC article. No abstract available.
References
-
- Vogt, V. M. (1997) in Retroviruses, eds. Coffin, J. M., Hughes, S. H. & Varmus, H. E. (Cold Spring Harbor Lab. Press, Plainview, N.Y.), Vol. 1, pp. 27–69.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

