The catalytic cycle of cytochrome c oxidase is not the sum of its two halves
- PMID: 14699047
- PMCID: PMC327181
- DOI: 10.1073/pnas.0306036101
The catalytic cycle of cytochrome c oxidase is not the sum of its two halves
Abstract
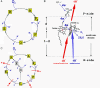
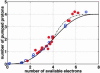
Membrane-bound cytochrome c oxidase catalyzes cell respiration in aerobic organisms and is a primary energy transducer in biology. The two halves of the catalytic cycle may be studied separately: in an oxidative phase, the enzyme is oxidized by O(2), and in a reductive phase, the oxidized enzyme is reduced before binding the next O(2) molecule. Here we show by time-resolved membrane potential and pH measurements with cytochrome oxidase liposomes that, with both phases in succession, two protons are translocated during each phase, one during each individual electron transfer step. However, when the reductive phase is not immediately preceded by oxidation, it follows a different reaction pathway no longer coupled to proton pumping. Metastable states with altered redox properties of the metal centers are accessed during turnover and relax when external electron donors are exhausted but recover after enzyme reduction and reoxidation by O(2). The efficiency of ATP synthesis might be regulated by switching between the two catalytic pathways.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

