Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity
- PMID: 14699049
- PMCID: PMC327176
- DOI: 10.1073/pnas.0307228101
Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity
Abstract
Defects in O-mannosylation of alpha-dystroglycan are thought to cause certain types of congenital muscular dystrophies with neuronal migration disorders. Among these muscular dystrophies, Walker-Warburg syndrome is caused by mutations in the gene encoding putative protein O-mannosyltransferase 1 (POMT1), which is homologous to yeast protein O-mannosyltransferases. However, there is no evidence that POMT1 has enzymatic activity. In this study, we first developed a method to detect protein O-mannosyltransferase activity in mammalian cells. Then, using this method, we showed that coexpression of both POMT1 and POMT2 (another gene homologous to yeast protein O-mannosyltransferases) was necessary for the enzyme activity, but expression of either POMT1 or POMT2 alone was insufficient. The requirement of an active enzyme complex of POMT1 and POMT2 suggests that the regulation of protein O-mannosylation is complex. Further, protein O-mannosylation appears to be required for normal structure and function of alpha-dystroglycan in muscle and brain. In view of the potential importance of this form of glycosylation for a number of developmental and neurobiological processes, the ability to assay mammalian protein O-mannosyltransferase activity should greatly facilitate progress in the identification and localization of O-mannosylated proteins and the elucidation of their functional roles.
Figures
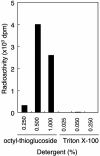
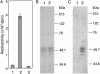

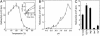
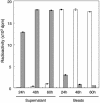
References
-
- Burton, E. A. & Davies, K. E. (2002) Cell 108, 5–8. - PubMed
-
- Emery, A. E. (2002) Lancet 359, 687–695. - PubMed
-
- Muntoni, F., Brockington, M., Blake, D. J., Torelli, S. & Brown, S. C. (2002) Lancet 360, 1419–1421. - PubMed
-
- Michele, D. E. & Campbell, K. P. (2003) J. Biol. Chem. 278, 15457–15460. - PubMed
-
- Holt, K. H., Crosbie, R. H., Venzke, D. P. & Campbell, K. P. (2000) FEBS Lett. 468, 79–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

