Repression of Na,K-ATPase beta1-subunit by the transcription factor snail in carcinoma
- PMID: 14699059
- PMCID: PMC363145
- DOI: 10.1091/mbc.e03-09-0646
Repression of Na,K-ATPase beta1-subunit by the transcription factor snail in carcinoma
Abstract
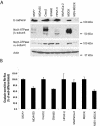
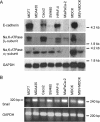
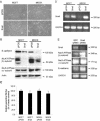
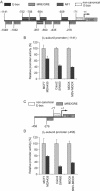
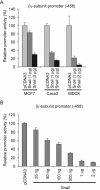
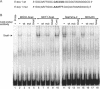
The Na,K-ATPase consists of two essential alpha- and beta-subunits and regulates the intracellular Na+ and K+ homeostasis. Although the alpha-subunit contains the catalytic activity, it is not active without functional beta-subunit. Here, we report that poorly differentiated carcinoma cell lines derived from colon, breast, kidney, and pancreas show reduced expression of the Na,K-ATPase beta1-subunit. Decreased expression of beta1-subunit in poorly differentiated carcinoma cell lines correlated with increased expression of the transcription factor Snail known to down-regulate E-cadherin. Ectopic expression of Snail in well-differentiated epithelial cell lines reduced the protein levels of E-cadherin and beta1-subunit and induced a mesenchymal phenotype. Reduction of Snail expression in a poorly differentiated carcinoma cell line by RNA interference increased the levels of Na,K-ATPase beta1-subunit. Furthermore, Snail binds to a noncanonical E-box in the Na,K-ATPase beta1-subunit promoter and suppresses its promoter activity. These results suggest that down-regulation of Na,K-ATPase beta1-subunit and E-cadherin by Snail are associated with events leading to epithelial to mesenchymal transition.
Figures
Similar articles
-
Na,K-ATPase β1-subunit is a target of sonic hedgehog signaling and enhances medulloblastoma tumorigenicity.Mol Cancer. 2015 Aug 19;14:159. doi: 10.1186/s12943-015-0430-1. Mol Cancer. 2015. PMID: 26286140 Free PMC article.
-
Regulation of Na,K-ATPase beta 1 subunit gene transcription by low external potassium in cardiac myocytes. Role of Sp1 AND Sp3.J Biol Chem. 2000 Aug 4;275(31):24173-84. doi: 10.1074/jbc.M002953200. J Biol Chem. 2000. PMID: 10811658
-
The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors.J Cell Sci. 2003 Feb 1;116(Pt 3):499-511. doi: 10.1242/jcs.00224. J Cell Sci. 2003. PMID: 12508111
-
Polarized membrane distribution of potassium-dependent ion pumps in epithelial cells: different roles of the N-glycans of their beta subunits.Cell Biochem Biophys. 2007;47(3):376-91. doi: 10.1007/s12013-007-0033-6. Cell Biochem Biophys. 2007. PMID: 17652782 Review.
-
Transcriptional regulators of Na,K-ATPase subunits.Front Cell Dev Biol. 2015 Oct 26;3:66. doi: 10.3389/fcell.2015.00066. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26579519 Free PMC article. Review.
Cited by
-
Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis.Mol Cell Biol. 2008 Mar;28(5):1528-40. doi: 10.1128/MCB.02061-07. Epub 2008 Jan 2. Mol Cell Biol. 2008. PMID: 18172008 Free PMC article.
-
Transcriptional factors associated with epithelial-mesenchymal transition in choroidal neovascularization.Mol Vis. 2011;17:1222-30. Epub 2011 May 6. Mol Vis. 2011. PMID: 21617757 Free PMC article.
-
Cancer as a channelopathy: ion channels and pumps in tumor development and progression.Front Cell Neurosci. 2015 Mar 17;9:86. doi: 10.3389/fncel.2015.00086. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25852478 Free PMC article. Review.
-
Hyaluronic acid/chitosan-g-poly(ethylene glycol) nanoparticles for gene therapy: an application for pDNA and siRNA delivery.Pharm Res. 2010 Dec;27(12):2544-55. doi: 10.1007/s11095-010-0263-y. Epub 2010 Sep 21. Pharm Res. 2010. PMID: 20857179
-
Na,K-ATPase β1-subunit is a target of sonic hedgehog signaling and enhances medulloblastoma tumorigenicity.Mol Cancer. 2015 Aug 19;14:159. doi: 10.1186/s12943-015-0430-1. Mol Cancer. 2015. PMID: 26286140 Free PMC article.
References
-
- Abbott, A., and Ball, W.J., Jr. (1993). The epitope for the inhibitory antibody M7-PB-E9 contains Ser-646 and Asp-652 of the sheep Na+, K(+)-ATPase alpha-subunit. Biochemistry 32, 3511-3518. - PubMed
-
- Batlle, E., Sancho, E., Franci, C., Dominguez, D., Monfar, M., Baulida, J., and Garcia De Herreros, A. (2000). The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2, 84-89. - PubMed
-
- Blanco, G., Melton, R.J., Sanchez, G., and Mercer, R.W. (1999). Functional characterization of a testes-specific alpha-subunit isoform of the sodium/potassium adenosinetriphosphatase. Biochemistry 38, 13661-13669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

