Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments
- PMID: 14699064
- PMCID: PMC363095
- DOI: 10.1091/mbc.e03-08-0581
Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments
Abstract
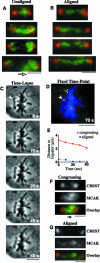
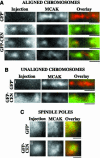
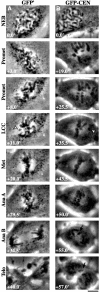
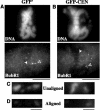
The complex behavior of chromosomes during mitosis is accomplished by precise binding and highly regulated polymerization dynamics of kinetochore microtubules. Previous studies have implicated Kin Is, unique kinesins that depolymerize microtubules, in regulating chromosome positioning. We have characterized the immunofluorescence localization of centromere-bound MCAK and found that MCAK localized to inner kinetochores during prophase but was predominantly centromeric by metaphase. Interestingly, MCAK accumulated at leading kinetochores during congression but not during segregation. We tested the consequences of MCAK disruption by injecting a centromere dominant-negative protein into prophase cells. Depletion of centromeric MCAK led to reduced centromere stretch, delayed chromosome congression, alignment defects, and severe missegregation of chromosomes. Rates of chromosome movement were unchanged, suggesting that the primary role of MCAK is not to move chromosomes. Furthermore, we found that disruption of MCAK leads to multiple kinetochore-microtubule attachment defects, including merotelic, syntelic, and combined merotelic-syntelic attachments. These findings reveal an essential role for Kin Is in prevention and/or correction of improper kinetochore-microtubule attachments.
Figures





Similar articles
-
Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity.Curr Biol. 2004 Feb 17;14(4):273-86. doi: 10.1016/j.cub.2004.01.055. Curr Biol. 2004. PMID: 14972678
-
A perikinetochoric ring defined by MCAK and Aurora-B as a novel centromere domain.PLoS Genet. 2006 Jun 2;2(6):e84. doi: 10.1371/journal.pgen.0020084. Epub 2006 Apr 20. PLoS Genet. 2006. PMID: 16741559 Free PMC article.
-
Chromosome congression in the absence of kinetochore fibres.Nat Cell Biol. 2009 Jul;11(7):832-8. doi: 10.1038/ncb1890. Epub 2009 Jun 14. Nat Cell Biol. 2009. PMID: 19525938 Free PMC article.
-
Merotelic kinetochores in mammalian tissue cells.Philos Trans R Soc Lond B Biol Sci. 2005 Mar 29;360(1455):553-68. doi: 10.1098/rstb.2004.1610. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 15897180 Free PMC article. Review.
-
Detection and correction of merotelic kinetochore orientation by Aurora B and its partners.Cell Cycle. 2007 Jul 1;6(13):1558-64. doi: 10.4161/cc.6.13.4452. Epub 2007 May 18. Cell Cycle. 2007. PMID: 17603301 Review.
Cited by
-
Chromosomal passengers: the four-dimensional regulation of mitotic events.Chromosoma. 2004 Nov;113(5):211-22. doi: 10.1007/s00412-004-0307-3. Epub 2004 Sep 4. Chromosoma. 2004. PMID: 15351889 Review.
-
Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference.Mol Biol Cell. 2005 Jul;16(7):3187-99. doi: 10.1091/mbc.e05-02-0167. Epub 2005 Apr 20. Mol Biol Cell. 2005. PMID: 15843429 Free PMC article.
-
Tetraploidy causes chromosomal instability in acentriolar mouse embryos.Nat Commun. 2019 Oct 23;10(1):4834. doi: 10.1038/s41467-019-12772-8. Nat Commun. 2019. PMID: 31645568 Free PMC article.
-
The influence of chromosome flexibility on chromosome transport during anaphase A.Proc Natl Acad Sci U S A. 2006 Apr 4;103(14):5349-54. doi: 10.1073/pnas.0601215103. Epub 2006 Mar 27. Proc Natl Acad Sci U S A. 2006. PMID: 16567616 Free PMC article.
-
Chromosome segregation machinery and cancer.Cancer Sci. 2009 Jul;100(7):1158-65. doi: 10.1111/j.1349-7006.2009.01178.x. Epub 2009 Apr 21. Cancer Sci. 2009. PMID: 19432891 Free PMC article. Review.
References
-
- Cassimeris, L., Rieder, C.L., Rupp, G., and Salmon, E.D. (1990). Stability of microtubule attachment to metaphase kinetochores in PtK1 cells. J. Cell Sci. 96, 9-15. - PubMed
-
- Cassimeris, L., Rieder, C.L., and Salmon, E.D. (1994). Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J. Cell Sci. 107, 285-297. - PubMed
-
- Cimini, D., Fioravanti, D., Salmon, E.D., and Degrassi, F. (2002). Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J. Cell Sci. 115, 507-515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

