Tumor necrosis factor-alpha promotes survival of opossum kidney cells via Cdc42-induced phospholipase C-gamma1 activation and actin filament redistribution
- PMID: 14699068
- PMCID: PMC363127
- DOI: 10.1091/mbc.e03-07-0491
Tumor necrosis factor-alpha promotes survival of opossum kidney cells via Cdc42-induced phospholipase C-gamma1 activation and actin filament redistribution
Abstract
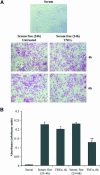
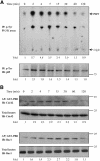
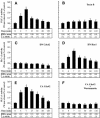
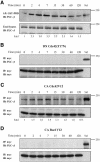
Although the renal proximal tubular epithelial cells are targeted in a variety of inflammatory diseases of the kidney, the signaling mechanism by which tumor necrosis factor (TNF)-alpha exerts its effects in these cells remains unclear. Here, we report that TNF-alpha elicits antiapoptotic effects in opossum kidney cells and that this response is mediated via actin redistribution through a novel signaling mechanism. More specifically, we show that TNF-alpha prevents apoptosis by inhibiting the activity of caspase-3 and this effect depends on actin polymerization state and nuclear factor-kappaB activity. We also demonstrate that the signaling cascade triggered by TNF-alpha is governed by the phosphatidylinositol-3 kinase, Cdc42/Rac1, and phospholipase (PLC)-gamma1. In this signaling cascade, Cdc42 was found to be selectively essential for PLC-gamma1 activation, whereas phosphatidylinositol-3,4,5-triphosphate alone is not sufficient to activate the phospholipase. Moreover, PLC-gamma1 was found to associate in vivo with the small GTPase(s). Interestingly, PLC-gamma1 was observed to associate with constitutively active (CA) Cdc42V12, but not with CA Rac1V12, whereas no interaction was detected with Cdc42(T17N). The inactive Cdc42(T17N) and the PLC-gamma1 inhibitor U73122 prevented actin redistribution and depolymerization, confirming that both signaling molecules are responsible for the reorganization of actin. Additionally, the actin filament stabilizer phallacidin potently blocked the nuclear translocation of nuclear factor-kappaB and its binding activity, resulting in abrogation of the TNF-alpha-induced inhibition of caspase-3. To conclude, our findings suggest that actin may play a pivotal role in the response of opossum kidney cells to TNF-alpha and implicate Cdc42 in directly regulating PLC-gamma1 activity.
Figures





Similar articles
-
PLC-gamma1 signaling pathway and villin activation are involved in actin cytoskeleton reorganization induced by Na+/Pi cotransport up-regulation.Mol Med. 2000 Apr;6(4):303-18. Mol Med. 2000. PMID: 10949911 Free PMC article.
-
Proteolytic cleavage of phospholipase C-gamma1 during apoptosis in Molt-4 cells.FASEB J. 2000 Jun;14(9):1083-92. doi: 10.1096/fasebj.14.9.1083. FASEB J. 2000. PMID: 10834929
-
Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization.Mol Biol Cell. 2002 Aug;13(8):2946-62. doi: 10.1091/mbc.02-01-0599. Mol Biol Cell. 2002. PMID: 12181358 Free PMC article.
-
[Sensitization of human glioma SWO cell line to tumor necrosis factor-induced apoptosis by blocking phospholipase C-gamma1 signaling pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2006 Mar;26(3):266-9. Nan Fang Yi Ke Da Xue Xue Bao. 2006. PMID: 16546723 Chinese.
-
Phospholipase C-gamma1 in tumor progression.Clin Exp Metastasis. 2003;20(4):285-90. doi: 10.1023/a:1024088922957. Clin Exp Metastasis. 2003. PMID: 12856715 Review.
Cited by
-
Up-regulation of Orai1 expression and store operated Ca(2+) entry following activation of membrane androgen receptors in MCF-7 breast tumor cells.BMC Cancer. 2015 Dec 21;15:995. doi: 10.1186/s12885-015-2014-2. BMC Cancer. 2015. PMID: 26690689 Free PMC article.
-
GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability.J Biol Chem. 2009 Apr 24;284(17):11454-66. doi: 10.1074/jbc.M805933200. Epub 2009 Mar 3. J Biol Chem. 2009. PMID: 19261619 Free PMC article.
-
TRAF2 facilitates vaccinia virus replication by promoting rapid virus entry.J Virol. 2014 Apr;88(7):3664-77. doi: 10.1128/JVI.03013-13. Epub 2014 Jan 15. J Virol. 2014. PMID: 24429366 Free PMC article.
-
Perturbation of the Actin Cytoskeleton in Human Hepatoma Cells Influences Interleukin-6 (IL-6) Signaling, but Not Soluble IL-6 Receptor Generation or NF-κB Activation.Int J Mol Sci. 2021 Jul 2;22(13):7171. doi: 10.3390/ijms22137171. Int J Mol Sci. 2021. PMID: 34281231 Free PMC article.
-
Pathogenic role of NF-kappaB activation in tubulointerstitial inflammatory lesions in human lupus nephritis.J Histochem Cytochem. 2008 May;56(5):517-29. doi: 10.1369/jhc.7A7368.2008. Epub 2008 Feb 18. J Histochem Cytochem. 2008. PMID: 18285351 Free PMC article.
References
-
- Are, A.F., V. E., Galkin, T. V. Pospelova, and G. P. Pinaev. (2000). The p65/RelA subunit of NF-kappaB interacts with actin-containing structures. Exp. Cell Res. 256, 533-544. - PubMed
-
- Auger, K.R., Serunian, L.A., Soltoff, S.P., Libby, P., and Cantley, C. (1989). PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57, 167-175. - PubMed
-
- Bae, Y.S., Cantley, L.G., Chen, C.S., Kim, S.R., Kwon, K.S., and Rhe, S.G. (1998). Activation of phospholipase C-gamma by phosphatidylinositol 3, 4, 5-trisphosphate. J. Biol. Chem. 273, 4465-4469. - PubMed
-
- Bagrodia, S., Taylor, S.J., Creasy, C.L., Chernoff, J., and Cerione, R.A. (1995). Identification of a mouse p21Cdc42/Rac-activated kinase. J. Biol. Chem. 270, 22731-22737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

