ago1 and dcr1, two core components of the RNA interference pathway, functionally diverge from rdp1 in regulating cell cycle events in Schizosaccharomyces pombe
- PMID: 14699070
- PMCID: PMC363162
- DOI: 10.1091/mbc.e03-06-0433
ago1 and dcr1, two core components of the RNA interference pathway, functionally diverge from rdp1 in regulating cell cycle events in Schizosaccharomyces pombe
Abstract
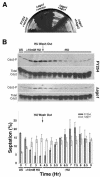
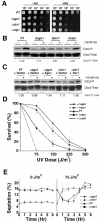
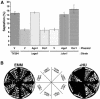
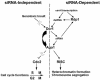
In the fission yeast Schizosaccharomyces pombe, three genes that function in the RNA interference (RNAi) pathway, ago1+, dcr1+, and rdp1+, have recently been shown to be important for timely formation of heterochromatin and accurate chromosome segregation. In the present study, we present evidence that null mutants for ago1+ and dcr1+ but not rdp1+, exhibit abnormal cytokinesis, cell cycle arrest deficiencies, and mating defects. Subsequent analyses showed that ago1+ and dcr1+ are required for regulated hyperphosphorylation of Cdc2 when encountering genotoxic insults. Because rdp1+ is dispensable for this process, the functions of ago1+ and dcr1+ in this pathway are presumably independent of their roles in RNAi-mediated heterochromatin formation and chromosome segregation. This was further supported by the finding that ago1+ is a multicopy suppressor of the S-M checkpoint deficiency and cytokinesis defects associated with loss of Dcr1 function, but not for the chromosome segregation defects of this mutant. Accordingly, we conclude that Dcr1-dependent production of small interfering RNAs is not required for enactment and/or maintenance of certain cell cycle checkpoints and that Ago1 and Dcr1 functionally diverge from Rdp1 to control cell cycle events in fission yeast. Finally, exogenous expression of hGERp95/EIF2C2/hAgo2, a human Ago1 homolog implicated in posttranscriptional gene silencing, compensated for the loss of ago1+ function in S. pombe. This suggests that PPD proteins may also be important for regulation of cell cycle events in higher eukaryotes.
Figures
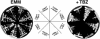
Similar articles
-
RNA interference effector proteins localize to mobile cytoplasmic puncta in Schizosaccharomyces pombe.Traffic. 2006 Aug;7(8):1032-44. doi: 10.1111/j.1600-0854.2006.00441.x. Epub 2006 May 25. Traffic. 2006. PMID: 16734665
-
The karyopherin Sal3 is required for nuclear import of the core RNA interference pathway protein Rdp1.Traffic. 2012 Apr;13(4):520-31. doi: 10.1111/j.1600-0854.2012.01333.x. Epub 2012 Feb 16. Traffic. 2012. PMID: 22268381
-
RNA interference regulates the cell cycle checkpoint through the RNA export factor, Ptr1, in fission yeast.Biochem Biophys Res Commun. 2012 Oct 12;427(1):143-7. doi: 10.1016/j.bbrc.2012.09.027. Epub 2012 Sep 16. Biochem Biophys Res Commun. 2012. PMID: 22989756
-
Studies on the mechanism of RNAi-dependent heterochromatin assembly.Cold Spring Harb Symp Quant Biol. 2006;71:461-71. doi: 10.1101/sqb.2006.71.044. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381328 Review.
-
Involvement of Dcr1 in post-transcriptional regulation of gene expression in Schizosaccharomyces pombe.Front Biosci. 2008 Jan 1;13:2203-15. doi: 10.2741/2835. Front Biosci. 2008. PMID: 17981703 Free PMC article. Review.
Cited by
-
Effect of betulinic acid on the regulation of Hiwi and cyclin B1 in human gastric adenocarcinoma AGS cells.Acta Pharmacol Sin. 2010 Jan;31(1):66-72. doi: 10.1038/aps.2009.177. Epub 2009 Dec 28. Acta Pharmacol Sin. 2010. PMID: 20037601 Free PMC article.
-
Characterizing the role of RNA silencing components in Cryptococcus neoformans.Fungal Genet Biol. 2010 Dec;47(12):1070-80. doi: 10.1016/j.fgb.2010.10.005. Epub 2010 Nov 9. Fungal Genet Biol. 2010. PMID: 21067947 Free PMC article.
-
Selection and characterization of RNA interference-deficient trypanosomes impaired in target mRNA degradation.Eukaryot Cell. 2004 Dec;3(6):1445-53. doi: 10.1128/EC.3.6.1445-1453.2004. Eukaryot Cell. 2004. PMID: 15590819 Free PMC article.
-
Aberrant T cell differentiation in the absence of Dicer.J Exp Med. 2005 Jul 18;202(2):261-9. doi: 10.1084/jem.20050678. Epub 2005 Jul 11. J Exp Med. 2005. PMID: 16009718 Free PMC article.
-
Conserved chromosomal functions of RNA interference.Nat Rev Genet. 2020 May;21(5):311-331. doi: 10.1038/s41576-019-0203-6. Epub 2020 Feb 12. Nat Rev Genet. 2020. PMID: 32051563 Free PMC article. Review.
References
-
- Alfa, C., Fantes, P., Hyams, J., McLeod, M., and Warbrick, E. (1993). Experiments with Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
-
- Allshire, R. (2002). Molecular biology. RNAi and heterochromatin - a hushed-up affair. Science 297, 1818-1819. - PubMed
-
- Bahler, J., Wu, J.Q., Longtine, M.S., Shah, N.G., McKenzie, A., 3rd, Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. (1998). Heterologous modules for efficient and versatile P.C.R-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

