In intact mammalian photoreceptors, Ca2+-dependent modulation of cGMP-gated ion channels is detectable in cones but not in rods
- PMID: 14699078
- PMCID: PMC2217411
- DOI: 10.1085/jgp.200308952
In intact mammalian photoreceptors, Ca2+-dependent modulation of cGMP-gated ion channels is detectable in cones but not in rods
Abstract
In the mammalian retina, cone photoreceptors efficiently adapt to changing background light intensity and, therefore, are able to signal small differences in luminance between objects and backgrounds, even when the absolute intensity of the background changes over five to six orders of magnitude. Mammalian rod photoreceptors, in contrast, adapt very little and only at intensities that nearly saturate the amplitude of their photoresponse. In search of a molecular explanation for this observation we assessed Ca2+-dependent modulation of ligand sensitivity in cyclic GMP-gated (CNG) ion channels of intact mammalian rods and cones. Solitary photoreceptors were isolated by gentle proteolysis of ground squirrel retina. Rods and cones were distinguished by whether or not their outer segments bind PNA lectin. We measured membrane currents under voltage-clamp in photoreceptors loaded with Diazo-2, a caged Ca2+ chelator, and fixed concentrations of 8Br-cGMP. At 600 nM free cytoplasmic Ca2+ the midpoint of the cone CNG channels sensitivity to 8BrcGMP, 8BrcGMPK1/2, is approximately 2.3 microM. The ligand sensitivity is less in rod than in cone channels. Instantly decreasing cytoplasmic Ca2+ to <30 nM activates a large inward membrane current in cones, but not in rods. Current activation arises from a Ca2+ -dependent modulation of cone CNG channels, presumably because of an increase in their affinity to the cyclic nucleotide. The time course of current activation is temperature dependent; it is well described by a single exponential process of approximately 480 ms time constant at 20-21 degrees C and 138 ms at 32 degrees C. The absence of detectable Ca2+-dependent CNG current modulation in intact rods, in view of the known channel modulation by calmodulin in-vitro, affirms the modulation in intact rods may only occur at low Ca2+ concentrations, those expected at intensities that nearly saturate the rod photoresponse. The correspondence between Ca2+ dependence of CNG modulation and the ability to light adapt suggest these events are correlated in photoreceptors.
Figures
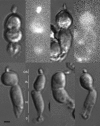


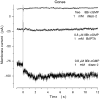


Similar articles
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
In intact cone photoreceptors, a Ca2+-dependent, diffusible factor modulates the cGMP-gated ion channels differently than in rods.J Gen Physiol. 1998 Nov;112(5):537-48. doi: 10.1085/jgp.112.5.537. J Gen Physiol. 1998. PMID: 9806963 Free PMC article.
-
Permeability and interaction of Ca2+ with cGMP-gated ion channels differ in retinal rod and cone photoreceptors.Biophys J. 1995 Jul;69(1):120-7. doi: 10.1016/S0006-3495(95)79881-2. Biophys J. 1995. PMID: 7545443 Free PMC article.
-
Differences in calcium homeostasis between retinal rod and cone photoreceptors revealed by the effects of voltage on the cGMP-gated conductance in intact cells.J Gen Physiol. 1994 Nov;104(5):909-40. doi: 10.1085/jgp.104.5.909. J Gen Physiol. 1994. PMID: 7876828 Free PMC article.
-
Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+ clearance rates.Cell Calcium. 1995 Oct;18(4):285-300. doi: 10.1016/0143-4160(95)90025-x. Cell Calcium. 1995. PMID: 8556768 Review.
Cited by
-
Temporal sampling, resetting, and adaptation orchestrate gradient sensing in sperm.J Cell Biol. 2012 Sep 17;198(6):1075-91. doi: 10.1083/jcb.201204024. J Cell Biol. 2012. PMID: 22986497 Free PMC article.
-
Disease-associated mutations in CNGB3 promote cytotoxicity in photoreceptor-derived cells.Mol Vis. 2013 Jun 11;19:1268-81. doi: 10.1167/13.9.1268. Print 2013. Mol Vis. 2013. PMID: 23805033 Free PMC article.
-
Calcium sets the physiological value of the dominant time constant of saturated mouse rod photoresponse recovery.PLoS One. 2010 Sep 27;5(9):e13025. doi: 10.1371/journal.pone.0013025. PLoS One. 2010. PMID: 20885958 Free PMC article.
-
The pharmacology of cyclic nucleotide-gated channels: emerging from the darkness.Curr Pharm Des. 2006;12(28):3597-613. doi: 10.2174/138161206778522100. Curr Pharm Des. 2006. PMID: 17073662 Free PMC article. Review.
-
A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to synaptic ribbons.J Neurosci. 2013 Jun 19;33(25):10278-300. doi: 10.1523/JNEUROSCI.5048-12.2013. J Neurosci. 2013. PMID: 23785143 Free PMC article.
References
-
- Adams, S.R., J.P.Y. Kao, and R.Y. Tsien. 1989. Biologically useful chelators that take up Ca2+ upon illumination. J. Am. Chem. Soc. 111:7957–7965.
-
- Anderson, D.H., and S.K. Fisher. 1976. The photoreceptors of diurnal squirrels: outer segment structure, disc shedding, and protein renewal. J. Ultrastruct. Res. 55:119–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

