Interaction between amino propeptides of type XI procollagen alpha1 chains
- PMID: 14699108
- PMCID: PMC2952413
- DOI: 10.1074/jbc.M310291200
Interaction between amino propeptides of type XI procollagen alpha1 chains
Abstract
Type XI collagen is a quantitatively minor yet essential constituent of the cartilage extracellular matrix. The amino propeptide of the alpha1 chain remains attached to the rest of the molecule for a longer period of time after synthesis than the other amino propeptides of type XI collagen and has been localized to the surface of thin collagen fibrils. Yeast two-hybrid system was used to demonstrate that a homodimer of alpha1(XI) amino propeptide (alpha1(XI)Npp) could form in vivo. Interaction was also confirmed using multi-angle laser light scattering, detecting an absolute weight average molar mass ranging from the size of a monomer to the size of a dimer (25,000-50,000 g/mol), respectively. Binding was shown to be saturable by ELISA. An interaction between recombinant alpha1(XI)Npp and the endogenous alpha1(XI)Npp was observed, and specificity for alpha1(XI)Npp but not alpha2(XI)Npp was demonstrated by co-precipitation. The interaction between the recombinant form of alpha1(XI)Npp and the endogenous alpha1(XI)Npp resulted in a stable association during the regeneration of cartilage extracellular matrix by fetal bovine chondrocytes maintained in pellet culture, generating a protein that migrated with an apparent molecular mass of 50-60 kDa on an SDS-polyacrylamide gel.
Figures

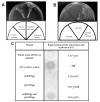


 ). Error bars indicate ± S.E.
). Error bars indicate ± S.E.
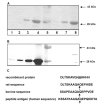

References
-
- Eyre DE, Wu JJ. In: Structure and Function of Collagen Types. Mayne R, Burgeson RE, editors. Academic Press, Inc.; Orlando, FL: 1987. pp. 261–309.
-
- Smith GN, Williams JM, Brandt KD. Collagen Relat. Res. 1987;7:17–25. - PubMed
-
- Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, Oxford JT, Morris NP, Andrikopoulos K, Ramirez F, Wardell BB, Lifferth GD, Teuscher C, Woodward SR, Taylor BA, Seegmiller RE, Olsen BR. Cell. 1995;80:423–430. - PubMed
-
- Eikenberry EF, Mendler M, Burgin R, Winterhalter KH, Bruckner P. In: Articular Cartilage and Osteoarthritis. Kuettner KE, Schleyerbach R, Peyron JG, Hascall VC, editors. Raven Press; New York: 1982. pp. 133–149.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

