Stem cells and the formation of the myocardium in the vertebrate embryo
- PMID: 14699629
- PMCID: PMC3096003
- DOI: 10.1002/ar.a.10130
Stem cells and the formation of the myocardium in the vertebrate embryo
Abstract
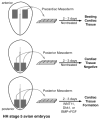
A major goal in cardiovascular biology is to repair diseased or damaged hearts with newly generated myocardial tissue. Stem cells offer a potential source of replacement myocytes for restoring cardiac function. Yet little is known about the nature of the cells that are able to generate myocardium and the conditions they require to form heart tissue. A source of information that may be pertinent to addressing these issues is the study of how the myocardium arises from progenitor cells in the early vertebrate embryo. Accordingly, this review will examine the initial events of cardiac developmental biology for insights into the identity and characteristics of the stem cells that can be used to generate myocardial tissue for therapeutic purposes.
Copyright 2004 Wiley-Liss, Inc.
Figures



References
-
- Alsan BH, Schultheiss TM. Regulation of avian cardiogenesis by Fgf8 signaling. Development. 2002;129:1935–1943. - PubMed
-
- Antin PB, Taylor RG, Yatskievych T. Precardiac mesoderm is specified during gastrulation in quail. Dev Dyn. 1994;200:144–154. - PubMed
-
- Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. - PubMed
-
- Beltrami AP, Urbanek K, Kajstura J, Yan S-M, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. - PubMed
-
- Biben C, Hatzistavrou T, Harvey RP. Expression of NK-2 class homeobox gene Nkx2-6 in foregut endoderm and heart. Mech Dev. 1998;73:125–127. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

