Early glomerular filtration defect and severe renal disease in podocin-deficient mice
- PMID: 14701729
- PMCID: PMC343810
- DOI: 10.1128/MCB.24.2.550-560.2004
Early glomerular filtration defect and severe renal disease in podocin-deficient mice
Abstract
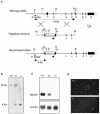
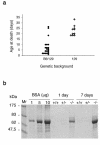
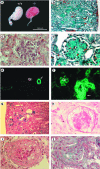
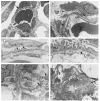
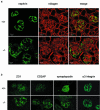
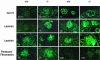
Podocytes are specialized epithelial cells covering the basement membrane of the glomerulus in the kidney. The molecular mechanisms underlying the role of podocytes in glomerular filtration are still largely unknown. We generated podocin-deficient (Nphs2-/-) mice to investigate the function of podocin, a protein expressed at the insertion of the slit diaphragm in podocytes and defective in a subset of patients with steroid-resistant nephrotic syndrome and focal and segmental glomerulosclerosis. Nphs2-/- mice developed proteinuria during the antenatal period and died a few days after birth from renal failure caused by massive mesangial sclerosis. Electron microscopy revealed the extensive fusion of podocyte foot processes and the lack of a slit diaphragm in the remaining foot process junctions. Using real-time PCR and immunolabeling, we showed that the expression of other slit diaphragm components was modified in Nphs2-/- kidneys: the expression of the nephrin gene was downregulated, whereas that of the ZO1 and CD2AP genes appeared to be upregulated. Interestingly, the progression of the renal disease, as well as the presence or absence of renal vascular lesions, depends on the genetic background. Our data demonstrate the crucial role of podocin in the establishment of the glomerular filtration barrier and provide a suitable model for mapping and identifying modifier genes involved in glomerular diseases caused by podocyte injuries.
Figures
References
-
- Bidani, A. K., K. A. Griffin, W. Plott, and M. M. Schwartz. 1993. Genetic predisposition to hypertension and microvascular injury in the remnant kidney model. J. Lab. Clin. Med. 122:284-291. - PubMed
-
- Boute, N., O. Gribouval, S. Roselli, F. Benessy, H. Lee, A. Fuchshuber, K. Dahan, M. C. Gubler, P. Niaudet, and C. Antignac. 2000. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24:349-354. - PubMed
-
- Cai, Y., A. Beziau, M. Sich, M. M. Kleppel, and M. C. Gubler. 1996. Collagen distribution in human membranous glomerulonephritis. Pediatr. Nephrol. 10:14-21. - PubMed
-
- Caridi, G., R. Bertelli, A. Carrea, M. Di Duca, P. Catarsi, M. Artero, M. Carraro, C. Zennaro, G. Candiano, L. Musante, M. Seri, F. Ginevri, F. Perfumo, and G. M. Ghiggeri. 2001. Prevalence, genetics, and clinical features of patients carrying podocin mutations in steroid-resistant nonfamilial focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 12:2742-2746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
