Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells
- PMID: 14701753
- PMCID: PMC343802
- DOI: 10.1128/MCB.24.2.823-836.2004
Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells
Abstract
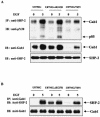
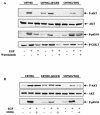
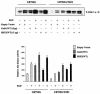
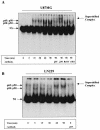
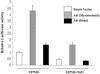
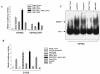
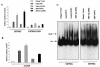
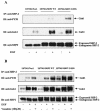
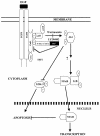
The transcription factor nuclear factor kappaB (NF-kappaB) plays an important role in inflammation and cancer, is activated by a variety of stimuli including tumor necrosis factor alpha, interleukin-1, UV irradiation, and viruses, as well as receptor tyrosine kinases, such as epidermal growth factor receptor (EGFR). Although previous studies suggest that EGFR can induce NF-kappaB, the mechanism of this activation remains unknown. In this study, we identify the components of the EGFR-induced signalosome in human glioblastoma cells required to regulate NF-kappaB activation. Immunoprecipitation analyses with ErbB-modulated cells indicate that association between SHP-2 and Grb2-associated binder 1 (Gab1) is the critical step in the formation of the signalosome linking EGFR to NF-kappaB activation. We also show that EGFR-induced NF-kappaB activation is mediated by the PI3-kinase/Akt activation loop. Overexpression of SHP-2, Gab1, and myristoylated Akt significantly upregulated NF-kappaB transcriptional activity and DNA binding activity in glioblastoma cells. Interestingly, overexpression of either one of the two SH2 domain mutants of SHP-2, R32E or R138E, slightly reduced NF-kappaB activity relative to that of wild-type SHP-2, indicating that the SH2 domains of SHP-2 are required for EGFR-induced NF-kappaB activation. On the other hand, ectopic overexpression of either a Gab1 mutant incapable of binding to SHP-2 (Y627F) or a phosphatase-inactive SHP-2 mutant (C459S) caused a significant increase in NF-kappaB activity. Moreover, SHP-2 C459S-expressing cells displayed higher Gab1 phosphotyrosine content, suggesting that SHP-2 regulates Gab1 phosphorylation through its phosphatase domain, which confers a negative regulatory effect on NF-kappaB activity. These results indicate that SHP-2/Gab1 association is critical for linking EGFR to NF-kappaB transcriptional activity via the PI3-kinase/Akt signaling axis in glioblastoma cells and that SHP-2 acts as a dual regulator of NF-kappaB activation.
Figures

References
-
- Arrandale, J. M., A. Gore-Willse, S. Rocks, J. M. Ren, J. Zhu, A. Davis, J. N. Livingston, and D. U. Rabin. 1996. Insulin signaling in mice expressing reduced levels of Syp. J. Biol. Chem. 271:21353-21358. - PubMed
-
- Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12:141-179. - PubMed
-
- Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. - PubMed
-
- Basile, J. R., A. Eichten, V. Zacny, and K. Munger. 2003. NF-kappaB-mediated induction of p21(Cip1/Waf1) by tumor necrosis factor alpha induces growth arrest and cytoprotection in normal human keratinocytes. Mol. Cancer Res. 1:262-270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
