Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice
- PMID: 14701760
- PMCID: PMC343815
- DOI: 10.1128/MCB.24.2.899-911.2004
Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice
Abstract
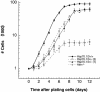
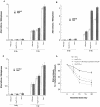
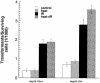
Heat shock proteins (HSPs) are highly conserved among all organisms from prokaryotes to eukaryotes. In mice, the HSP genes Hsp70.1 and Hsp70.3 are induced by both endogenous and exogenous stressors, such as heat and toxicants. In order to determine whether such proteins specifically influence genomic instability, mice deficient for Hsp70.1 and Hsp70.3 (Hsp70.1/3(-/-) mice) were generated by gene targeting. Mouse embryonic fibroblasts (MEFs) prepared from Hsp70.1/3(-/-) mice did not synthesize Hsp70.1 or Hsp70.3 after heat-induced stress. While the Hsp70.1/3(-/-) mutant mice were fertile, their cells displayed genomic instability that was enhanced by heat treatment. Cells from Hsp70.1/3(-/-) mice also display a higher frequency of chromosome end-to-end associations than do control Hsp70.1/3(+/+) cells. To determine whether observed genomic instability was related to defective chromosome repair, Hsp70.1/3(-/-) and Hsp70.1/3(+/+) fibroblasts were treated with ionizing radiation (IR) alone or heat and IR. Exposure to IR led to more residual chromosome aberrations, radioresistant DNA synthesis (a hallmark of genomic instability), increased cell killing, and enhanced IR-induced oncogenic transformation in Hsp70.1/3(-/-) cells. Heat treatment prior to IR exposure enhanced cell killing, S-phase-specific chromosome damage, and the frequency of transformants in Hsp70.1/3(-/-) cells in comparison to Hsp70.1/3(+/+) cells. Both in vivo and in vitro studies demonstrate for the first time that Hsp70.1 and Hsp70.3 have an essential role in maintaining genomic stability under stress conditions.
Figures







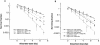
References
-
- Barker, K., M. Khayat, N. Miller, M. Wilson, L. W. Clem, and E. Bengten. 2002. Immortal and mortal clonal lymphocyte lines from channel catfish: comparison of telomere length, telomerase activity, tumor suppressor and heat shock protein expression. Dev. Comp. Immunol. 26:45-51. - PubMed
-
- Bercovich, B., I. Stancovski, A. Mayer, N. Blumenfeld, A. Laszlo, A. L. Schwartz, and A. Ciechanover. 1997. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J. Biol. Chem. 272:9002-9010. - PubMed
-
- Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. - PubMed
-
- Bukau, B., E. Deuerling, C. Pfund, and E. A. Craig. 2000. Getting newly synthesized proteins into shape. Cell 101:119-122. - PubMed
-
- Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92:351-366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
