Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction
- PMID: 14701872
- PMCID: PMC305254
- DOI: 10.1101/gad.1153603
Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction
Abstract
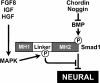
How do very diverse signaling pathways induce neural differentiation in Xenopus? Anti-BMP (Chordin), FGF8, and IGF2 signals are integrated in the embryo via the regulation of Smad1 phosphorylation. Neural induction results from the combined inhibition of BMP receptor serine/threonine kinases and activation of receptor tyrosine kinases that signal through MAPK and phosphorylate Smad1 in the linker region, further inhibiting Smad1 transcriptional activity. This hard-wired molecular mechanism at the level of the Smad1 transcription factor may help explain the opposing activities of IGF, FGF, and BMP signals not only in neural induction, but also in other aspects of vertebrate development.
Figures




Comment in
-
Integration of Smad and MAPK pathways: a link and a linker revisited.Genes Dev. 2003 Dec 15;17(24):2993-7. doi: 10.1101/gad.1167003. Genes Dev. 2003. PMID: 14701870 No abstract available.
Similar articles
-
Integration of Smad and MAPK pathways: a link and a linker revisited.Genes Dev. 2003 Dec 15;17(24):2993-7. doi: 10.1101/gad.1167003. Genes Dev. 2003. PMID: 14701870 No abstract available.
-
Evidence for antagonism of BMP-4 signals by MAP kinase during Xenopus axis determination and neural specification.Differentiation. 2003 Sep;71(7):434-44. doi: 10.1046/j.1432-0436.2003.7107006.x. Differentiation. 2003. PMID: 12969336
-
SANE, a novel LEM domain protein, regulates bone morphogenetic protein signaling through interaction with Smad1.J Biol Chem. 2003 Jan 3;278(1):428-37. doi: 10.1074/jbc.M210505200. Epub 2002 Oct 21. J Biol Chem. 2003. PMID: 12393873
-
Dorsal-ventral patterning and neural induction in Xenopus embryos.Annu Rev Cell Dev Biol. 2004;20:285-308. doi: 10.1146/annurev.cellbio.20.011403.154124. Annu Rev Cell Dev Biol. 2004. PMID: 15473842 Free PMC article. Review.
-
Active signals, gradient formation and regional specificity in neural induction.Exp Cell Res. 2014 Feb 1;321(1):25-31. doi: 10.1016/j.yexcr.2013.11.018. Epub 2013 Dec 4. Exp Cell Res. 2014. PMID: 24315941 Review.
Cited by
-
Requirement of the MEK5-ERK5 pathway for neural differentiation in Xenopus embryonic development.EMBO Rep. 2005 Nov;6(11):1064-9. doi: 10.1038/sj.embor.7400515. Epub 2005 Sep 23. EMBO Rep. 2005. PMID: 16179948 Free PMC article.
-
Forskolin and IBMX Induce Neural Transdifferentiation of MSCs Through Downregulation of the NRSF.Sci Rep. 2019 Feb 27;9(1):2969. doi: 10.1038/s41598-019-39544-0. Sci Rep. 2019. PMID: 30814572 Free PMC article.
-
Protein palmitoylation regulates osteoblast differentiation through BMP-induced osterix expression.PLoS One. 2009;4(1):e4135. doi: 10.1371/journal.pone.0004135. Epub 2009 Jan 6. PLoS One. 2009. PMID: 19125191 Free PMC article.
-
Intracellular Communication among Morphogen Signaling Pathways during Vertebrate Body Plan Formation.Genes (Basel). 2020 Mar 24;11(3):341. doi: 10.3390/genes11030341. Genes (Basel). 2020. PMID: 32213808 Free PMC article. Review.
-
MAPK and PI3K signaling: At the crossroads of neural crest development.Dev Biol. 2018 Dec 1;444 Suppl 1(Suppl 1):S79-S97. doi: 10.1016/j.ydbio.2018.02.003. Epub 2018 Feb 14. Dev Biol. 2018. PMID: 29453943 Free PMC article. Review.
References
-
- Blume-Jensen P. and Hunter, T. 2001. Oncogenic kinase signalling. Nature 411: 355-365. - PubMed
-
- Chesnel F., Bonnec, G., Tardivel, A., and Boujard, D. 1997. Comparative effects of insulin on the activation of the Raf/Mos-dependent MAP kinase cascade in vitellogenic versus postvitellogenic Xenopus oocytes. Dev. Biol. 188: 122-133. - PubMed
-
- Christen B. and Slack, J.M.W. 1997. FGF-8 is associated with anteroposterior patterning and limb regeneration in Xenopus. Dev. Biol. 192: 455-466. - PubMed
-
- Collavin L. and Kirschner, M.W. 2003. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development 130: 805-816. - PubMed
-
- Cooke J. and Smith, J.C. 1989. Gastrulation and larval pattern in Xenopus after blastocoelic injection of a Xenopus-derived inducing factor: Experiments testing models for the normal organization of mesoderm. Dev. Biol. 131: 383-400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
