Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation
- PMID: 14701912
- PMCID: PMC316311
- DOI: 10.1104/pp.103.027888
Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation
Abstract
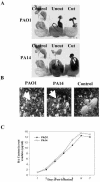
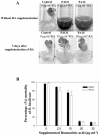
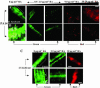
Pseudomonas aeruginosa is an opportunistic human pathogen capable of forming a biofilm under physiological conditions that contributes to its persistence despite long-term treatment with antibiotics. Here, we report that pathogenic P. aeruginosa strains PAO1 and PA14 are capable of infecting the roots of Arabidopsis and sweet basil (Ocimum basilicum), in vitro and in the soil, and are capable of causing plant mortality 7 d postinoculation. Before plant mortality, PAO1 and PA14 colonize the roots of Arabidopsis and sweet basil and form a biofilm as observed by scanning electron microscopy, phase contrast microscopy, and confocal scanning laser microscopy. Upon P. aeruginosa infection, sweet basil roots secrete rosmarinic acid (RA), a multifunctional caffeic acid ester that exhibits in vitro antibacterial activity against planktonic cells of both P. aeruginosa strains with a minimum inhibitory concentration of 3 microg mL(-1). However, in our studies RA did not attain minimum inhibitory concentration levels in sweet basil's root exudates before P. aeruginosa formed a biofilm that resisted the microbicidal effects of RA and ultimately caused plant mortality. We further demonstrated that P. aeruginosa biofilms were resistant to RA treatment under in vivo and in vitro conditions. In contrast, induction of RA secretion by sweet basil roots and exogenous supplementation of Arabidopsis root exudates with RA before infection conferred resistance to P. aeruginosa. Under the latter conditions, confocal scanning laser microscopy revealed large clusters of dead P. aeruginosa on the root surface of Arabidopsis and sweet basil, and biofilm formation was not observed. Studies with quorum-sensing mutants PAO210 (DeltarhlI), PAO214 (DeltalasI), and PAO216 (DeltalasI DeltarhlI) demonstrated that all of the strains were pathogenic to Arabidopsis, which does not naturally secrete RA as a root exudate. However, PAO214 was the only pathogenic strain toward sweet basil, and PAO214 biofilm appeared comparable with biofilms formed by wild-type strains of P. aeruginosa. Our results collectively suggest that upon root colonization, P. aeruginosa forms a biofilm that confers resistance against root-secreted antibiotics.
Figures





References
-
- Bais HP, Loyola Vargas VM, Flores HE, Vivanco JM (2001) Root-specific metabolism: the biology and biochemistry of underground organs. In Vitro Cell Dev Biol Plant 37: 730-741
-
- Bais HP, Walker TS, Schweizer HP, Vivanco JM (2002) Root-specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol Biochem 40: 983-995
-
- Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkoff-Schweizer RR, Schweizer HP (2001) Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants over expressing MexCD-OprJ. Antimicrob Agents Chemother 45: 428-432 - PMC - PubMed
-
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49: 711-745 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

