Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient
- PMID: 14702111
- PMCID: PMC300760
- DOI: 10.1172/JCI17005
Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient
Abstract
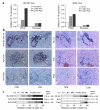
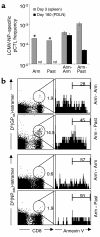
Viruses can cause but can also prevent autoimmune disease. This dualism has certainly hampered attempts to establish a causal relationship between viral infections and type 1 diabetes (T1D). To develop a better mechanistic understanding of how viruses can influence the development of autoimmune disease, we exposed prediabetic mice to various viral infections. We used the well-established NOD and transgenic RIP-LCMV models of autoimmune diabetes. In both cases, infection with the lymphocytic choriomeningitis virus (LCMV) completely abrogated the diabetic process. Interestingly, such therapeutic viral infections resulted in a rapid recruitment of T lymphocytes from the islet infiltrate to the pancreatic draining lymph node, where increased apoptosis was occurring. In both models this was associated with a selective and extensive expression of the chemokine IP-10 (CXCL10), which predominantly attracts activated T lymphocytes, in the pancreatic draining lymph node, and in RIP-LCMV mice it depended on the viral antigenic load. In RIP-LCMV mice, blockade of TNF-alpha or IFN-gamma in vivo abolished the prevention of T1D. Thus, virally induced proinflammatory cytokines and chemokines can influence the ongoing autoaggressive process beneficially at the preclinical stage, if produced at the correct location, time, and levels.
Figures




References
-
- Kallmann BA, et al. Cytokine secretion patterns in twins discordant for Type I diabetes. Diabetologia. 1999;42:1080–1085. - PubMed
-
- Honeyman MC, et al. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49:1319–1324. - PubMed
-
- Notkins, A.L., and Yoon, J.-W. 1984. Virus-induced diabetes mellitus. In Concepts in viral pathogenesis. A.L. Notkins and M.B.A. Oldstone, editors. Springer-Verlag. New York, New York, USA. 241–247.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

