Nitrite in saliva increases gastric mucosal blood flow and mucus thickness
- PMID: 14702114
- PMCID: PMC300767
- DOI: 10.1172/JCI19019
Nitrite in saliva increases gastric mucosal blood flow and mucus thickness
Erratum in
- J Clin Invest. 2004 Feb;113(3):490
Abstract
Salivary nitrate from dietary or endogenous sources is reduced to nitrite by oral bacteria. In the acidic stomach, nitrite is further reduced to NO and related compounds, which have potential biological activity. We used an in vivo rat model as a bioassay to test effects of human saliva on gastric mucosal blood flow and mucus thickness. Gastric mucosal blood flow and mucus thickness were measured after topical administration of human saliva in HCl. The saliva was collected either after fasting (low in nitrite) or after ingestion of sodium nitrate (high in nitrite). In additional experiments, saliva was exchanged for sodium nitrite at different doses. Mucosal blood flow was increased after luminal application of nitrite-rich saliva, whereas fasting saliva had no effects. Also, mucus thickness increased in response to nitrite-rich saliva. The effects of nitrite-rich saliva were similar to those of topically applied sodium nitrite. Nitrite-mediated effects were associated with generation of NO and S-nitrosothiols. In addition, pretreatment with an inhibitor of guanylyl cyclase markedly inhibited nitrite-mediated effects on blood flow. We conclude that nitrite-containing human saliva given luminally increases gastric mucosal blood flow and mucus thickness in the rat. These effects are likely mediated through nonenzymatic generation of NO via activation of guanylyl cyclase. This supports a gastroprotective role of salivary nitrate/nitrite.
Figures
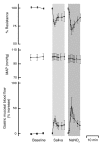




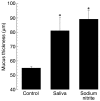

Comment in
-
Haldane, hot dogs, halitosis, and hypoxic vasodilation: the emerging biology of the nitrite anion.J Clin Invest. 2004 Jan;113(1):19-21. doi: 10.1172/JCI20664. J Clin Invest. 2004. PMID: 14702102 Free PMC article. Review.
References
-
- Cheung LY, Chang N. The role of gastric mucosal blood flow and H+ back-diffusion in the pathogenesis of acute gastric erosions. J. Surg. Res. 1977;22:357–361. - PubMed
-
- McGreevy JM, Moody FG. Protection of gastric mucosa against aspirin-induced erosions by enhanced blood flow. Surg. Forum. 1977;28:357–359. - PubMed
-
- Leung FW, Itoh M, Hirabayashi K, Guth PH. Role of blood flow in gastric and duodenal mucosal injury in the rat. Gastroenterology. 1985;88:281–289. - PubMed
-
- Stein HJ, Bauerfeind P, Hinder RA, Koerfer J, Blum AL. Luminal acid reduces gastric mucosal blood flow in the ischemic stomach. J. Surg. Res. 1989;46:616–619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

