Identification of the LIV-I/LS system as the third phenylalanine transporter in Escherichia coli K-12
- PMID: 14702302
- PMCID: PMC305776
- DOI: 10.1128/JB.186.2.343-350.2004
Identification of the LIV-I/LS system as the third phenylalanine transporter in Escherichia coli K-12
Abstract
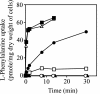
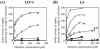
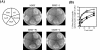
In Escherichia coli, the active transport of phenylalanine is considered to be performed by two different systems, AroP and PheP. However, a low level of accumulation of phenylalanine was observed in an aromatic amino acid transporter-deficient E. coli strain (DeltaaroP DeltapheP Deltamtr Deltatna DeltatyrP). The uptake of phenylalanine by this strain was significantly inhibited in the presence of branched-chain amino acids. Genetic analysis and transport studies revealed that the LIV-I/LS system, which is a branched-chain amino acid transporter consisting of two periplasmic binding proteins, the LIV-binding protein (LIV-I system) and LS-binding protein (LS system), and membrane components, LivHMGF, is involved in phenylalanine accumulation in E. coli cells. The K(m) values for phenylalanine in the LIV-I and LS systems were determined to be 19 and 30 micro M, respectively. Competitive inhibition of phenylalanine uptake by isoleucine, leucine, and valine was observed for the LIV-I system and, surprisingly, also for the LS system, which has been assumed to be leucine specific on the basis of the results of binding studies with the purified LS-binding protein. We found that the LS system is capable of transporting isoleucine and valine with affinity comparable to that for leucine and that the LIV-I system is able to transport tyrosine with affinity lower than that seen with other substrates. The physiological importance of the LIV-I/LS system for phenylalanine accumulation was revealed in the growth of phenylalanine-auxotrophic E. coli strains under various conditions.
Figures

References
-
- Adams, M. D., L. M. Wagner, T. J. Graddis, R. Landick, T. K. Antonucci, A. L. Gibson, and D. L. Oxender. 1990. Nucleotide sequence and genetic characterization reveal six essential genes for the LIV-I and LS transport systems of Escherichia coli. J. Biol. Chem. 265:11436-11443. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

