Dihydropteridine reductase as an alternative to dihydrofolate reductase for synthesis of tetrahydrofolate in Thermus thermophilus
- PMID: 14702303
- PMCID: PMC305743
- DOI: 10.1128/JB.186.2.351-355.2004
Dihydropteridine reductase as an alternative to dihydrofolate reductase for synthesis of tetrahydrofolate in Thermus thermophilus
Abstract
A strategy devised to isolate a gene coding for a dihydrofolate reductase from Thermus thermophilus DNA delivered only clones harboring instead a gene (the T. thermophilus dehydrogenase [DH(Tt)] gene) coding for a dihydropteridine reductase which displays considerable dihydrofolate reductase activity (about 20% of the activity detected with 6,7-dimethyl-7,8-dihydropterine in the quinonoid form as a substrate). DH(Tt) appears to account for the synthesis of tetrahydrofolate in this bacterium, since a classical dihydrofolate reductase gene could not be found in the recently determined genome nucleotide sequence (A. Henne, personal communication). The derived amino acid sequence displays most of the highly conserved cofactor and active-site residues present in enzymes of the short-chain dehydrogenase/reductase family. The enzyme has no pteridine-independent oxidoreductase activity, in contrast to Escherichia coli dihydropteridine reductase, and thus appears more similar to mammalian dihydropteridine reductases, which do not contain a flavin prosthetic group. We suggest that bifunctional dihydropteridine reductases may be responsible for the synthesis of tetrahydrofolate in other bacteria, as well as archaea, that have been reported to lack a classical dihydrofolate reductase but for which possible substitutes have not yet been identified.
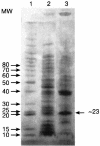
Figures


Similar articles
-
Dihydropteridine reductase from Escherichia coli exhibits dihydrofolate reductase activity.Biol Chem Hoppe Seyler. 1992 Oct;373(10):1067-73. doi: 10.1515/bchm3.1992.373.2.1067. Biol Chem Hoppe Seyler. 1992. PMID: 1418677
-
Is dihydropteridine reductase an anomalous dihydrofolate reductase, a flavin-like enzyme, or a short-chain dehydrogenase?Adv Exp Med Biol. 1993;338:115-21. doi: 10.1007/978-1-4615-2960-6_23. Adv Exp Med Biol. 1993. PMID: 8304093 Review. No abstract available.
-
Binding profile of quinonoid-dihydrobiopterin to quinonoid-dihydropteridine reductase examined by in silico and in vitro analyses.J Biochem. 2023 Oct 31;174(5):441-450. doi: 10.1093/jb/mvad062. J Biochem. 2023. PMID: 37540845
-
FolM, a new chromosomally encoded dihydrofolate reductase in Escherichia coli.J Bacteriol. 2003 Dec;185(23):7015-8. doi: 10.1128/JB.185.23.7015-7018.2003. J Bacteriol. 2003. PMID: 14617668 Free PMC article.
-
Comparative properties of three pteridine reductases.Adv Exp Med Biol. 1999;463:403-10. doi: 10.1007/978-1-4615-4735-8_50. Adv Exp Med Biol. 1999. PMID: 10352712 Review. No abstract available.
Cited by
-
Phylogenomic and functional analysis of pterin-4a-carbinolamine dehydratase family (COG2154) proteins in plants and microorganisms.Plant Physiol. 2008 Apr;146(4):1515-27. doi: 10.1104/pp.107.114090. Epub 2008 Feb 1. Plant Physiol. 2008. PMID: 18245455 Free PMC article.
-
Effect of simulated microgravity on oxidation-sensitive gene expression in PC12 cells.Adv Space Res. 2006;38(6):1168-1176. doi: 10.1016/j.asr.2006.02.059. Adv Space Res. 2006. PMID: 19081771 Free PMC article.
-
Genome-guided analysis of physiological and morphological traits of the fermentative acetate oxidizer Thermacetogenium phaeum.BMC Genomics. 2012 Dec 23;13:723. doi: 10.1186/1471-2164-13-723. BMC Genomics. 2012. PMID: 23259483 Free PMC article.
-
Flavin-dependent thymidylate synthase ThyX activity: implications for the folate cycle in bacteria.J Bacteriol. 2007 Dec;189(23):8537-45. doi: 10.1128/JB.01380-07. Epub 2007 Sep 21. J Bacteriol. 2007. PMID: 17890305 Free PMC article.
-
[NiFe] hydrogenase from Alteromonas macleodii with unusual stability in the presence of oxygen and high temperature.Appl Environ Microbiol. 2011 Mar;77(6):1990-8. doi: 10.1128/AEM.01559-10. Epub 2011 Jan 21. Appl Environ Microbiol. 2011. PMID: 21257809 Free PMC article.
References
-
- Di Fraia, R., W. Wilquet, M. A. Ciardello, V. Carratore, A. Antignani, L. Camardella, N. Glansdorff, and G. di Prisco. 2000. NADP+-dependent glutamate dehydrogenase in the Antarctic psychrotolerant bacterium Psychrobacter sp. TAD1. Characterization, protein and DNA sequence, and relationship to other glutamate dehydrogenases. Eur. J. Biochem. 267:121-131. - PubMed
-
- Hamm-Alvarez, S. F., A. Sancar, and K. V. Rajagopalan. 1990. The presence and distribution of reduced folates in Escherichia coli dihydrofolate reductase mutants. J. Biol. Chem. 265:9850-9856. - PubMed
-
- Hitchings, G. H., Jr. 1989. Nobel lecture in physiology or medicine, 1988: selective inhibitors of dihydrofolate reductase. In Vitro Cell. Dev. Biol. 25:303-310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

