Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression
- PMID: 14707114
- PMCID: PMC1887733
- DOI: 10.1084/jem.20031104
Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression
Abstract
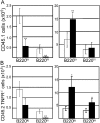
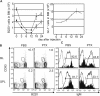
Inflammation removes developing and mature lymphocytes from the bone marrow (BM) and induces the appearance of developing B cells in the spleen. BM granulocyte numbers increase after lymphocyte reductions to support a reactive granulocytosis. Here, we demonstrate that inflammation, acting primarily through tumor necrosis factor alpha (TNFalpha), mobilizes BM lymphocytes. Mobilization reflects a reduced CXCL12 message and protein in BM and changes to the BM environment that prevents homing by cells from naive donors. The effects of TNFalpha are potentiated by interleukin 1 beta (IL-1beta), which acts primarily to expand the BM granulocyte compartment. Our observations indicate that inflammation induces lymphocyte mobilization by suppressing CXCL12 retention signals in BM, which, in turn, increases the ability of IL-1beta to expand the BM granulocyte compartment. Consistent with this idea, lymphocyte mobilization and a modest expansion of BM granulocyte numbers follow injections of pertussis toxin. We propose that TNFalpha and IL-1beta transiently specialize the BM to support acute granulocytic responses and consequently promote extramedullary lymphopoiesis.
Figures






Similar articles
-
Bone marrow B cell apoptosis during in vivo influenza virus infection requires TNF-alpha and lymphotoxin-alpha.J Immunol. 2002 Dec 1;169(11):6193-201. doi: 10.4049/jimmunol.169.11.6193. J Immunol. 2002. PMID: 12444124
-
Expression of stromal cell-derived factor-1/pre-B cell growth-stimulating factor receptor, CXC chemokine receptor 4, on CD34+ human bone marrow cells is a phenotypic alteration for committed lymphoid progenitors.J Immunol. 1999 Oct 1;163(7):3612-20. J Immunol. 1999. PMID: 10490954
-
Tumor necrosis factor receptor regulation of bone marrow cell apoptosis during endotoxin-induced systemic inflammation.Shock. 2006 May;25(5):464-71. doi: 10.1097/01.shk.0000209544.22048.02. Shock. 2006. PMID: 16680011
-
The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4.Eur J Immunol. 1997 Jul;27(7):1788-93. doi: 10.1002/eji.1830270729. Eur J Immunol. 1997. PMID: 9247593
-
Inflammation rapidly reorganizes mouse bone marrow B cells and their environment in conjunction with early IgM responses.Blood. 2015 Sep 3;126(10):1184-92. doi: 10.1182/blood-2015-03-635805. Epub 2015 Jul 13. Blood. 2015. PMID: 26170030
Cited by
-
In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors.Aging Cell. 2013 Apr;12(2):303-11. doi: 10.1111/acel.12055. Aging Cell. 2013. PMID: 23410004 Free PMC article.
-
CD23+ CD21(high) CD1d(high) B cells in inflamed lymph nodes are a locally differentiated population with increased antigen capture and activation potential.J Immunol. 2012 Jun 15;188(12):5944-53. doi: 10.4049/jimmunol.1103071. Epub 2012 May 16. J Immunol. 2012. PMID: 22593620 Free PMC article.
-
Mice Deficient in NOX2 Display Severe Thymic Atrophy, Lymphopenia, and Reduced Lymphopoiesis in a Zymosan-Induced Model of Systemic Inflammation.Inflammation. 2021 Feb;44(1):371-382. doi: 10.1007/s10753-020-01342-6. Epub 2020 Sep 16. Inflammation. 2021. PMID: 32939668
-
Macrophage-Lineage Cells Negatively Regulate the Hematopoietic Stem Cell Pool in Response to Interferon Gamma at Steady State and During Infection.Stem Cells. 2015 Jul;33(7):2294-305. doi: 10.1002/stem.2040. Epub 2015 May 22. Stem Cells. 2015. PMID: 25880153 Free PMC article.
-
Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization.J Exp Med. 2005 Feb 21;201(4):545-54. doi: 10.1084/jem.20042060. Epub 2005 Feb 14. J Exp Med. 2005. PMID: 15710653 Free PMC article.
References
-
- Young, N.S., B. Baranski, and G. Kurtzman. 1989. The immune system as mediator of virus-associated bone marrow failure: B19 parvovirus and Epstein-Barr virus. Ann. NY Acad. Sci. 554:75–80. - PubMed
-
- Apperley, J.F., C. Dowding, J. Hibbin, J. Buiter, E. Matutes, P.J. Sissons, M. Gordon, and J.M. Goldman. 1989. The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp. Hematol. 17:38–45. - PubMed
-
- Dorshkind, K. 1988. Interleukin-1 inhibition of B lymphopoiesis is reversible. Blood. 72:2053–2055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

