Interpretation of diagnostic laboratory tests for severe acute respiratory syndrome: the Toronto experience
- PMID: 14707219
- PMCID: PMC305313
Interpretation of diagnostic laboratory tests for severe acute respiratory syndrome: the Toronto experience
Abstract
Background: An outbreak of severe acute respiratory syndrome (SARS) began in Canada in February 2003. The initial diagnosis of SARS was based on clinical and epidemiological criteria. During the outbreak, molecular and serologic tests for the SARS-associated coronavirus (SARS-CoV) became available. However, without a "gold standard," it was impossible to determine the usefulness of these tests. We describe how these tests were used during the first phase of the SARS outbreak in Toronto and offer some recommendations that may be useful if SARS returns.
Methods: We examined the results of all diagnostic laboratory tests used in 117 patients admitted to hospitals in Toronto who met the Health Canada criteria for suspect or probable SARS. Focusing on tests for SARS-CoV, we attempted to determine the optimal specimen types and timing of specimen collection.
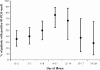
Results: Diagnostic test results for SARS-CoV were available for 110 of the 117 patients. SARS-CoV was detected by means of reverse-transcriptase polymerase chain reaction (RT-PCR) in at least one specimen in 59 (54.1%) of 109 patients. Serologic test results of convalescent samples were positive in 50 (96.2%) of 52 patients for whom paired serum samples were collected during the acute and convalescent phases of the illness. Of the 110 patients, 78 (70.9%) had specimens that tested positive by means of RT-PCR, serologic testing or both methods. The proportion of RT-PCR test results that were positive was similar between patients who met the criteria for suspect SARS (50.8%, 95% confidence interval [CI] 38.4%-63.2%) and those who met the criteria for probable SARS (58.0%, 95% CI 44.2%-70.7%). SARS-CoV was detected in nasopharyngeal swabs in 33 (32.4%) of 102 patients, in stool specimens in 19 (63.3%) of 30 patients, and in specimens from the lower respiratory tract in 10 (58.8%) of 17 patients.
Interpretation: These findings suggest that the rapid diagnostic tests in use at the time of the initial outbreak lack sufficient sensitivity to be used clinically to rule out SARS. As tests for SARS-CoV continue to be optimized, evaluation of the clinical presentation and elucidation of a contact history must remain the cornerstone of SARS diagnosis. In patients with SARS, specimens taken from the lower respiratory tract and stool samples test positive by means of RT-PCR more often than do samples taken from other areas.
Figures

Similar articles
-
Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada.CMAJ. 2003 Aug 19;169(4):285-92. CMAJ. 2003. PMID: 12925421 Free PMC article.
-
Severe acute respiratory syndrome-associated coronavirus in lung tissue.Emerg Infect Dis. 2004 Jan;10(1):20-4. doi: 10.3201/eid1001.030404. Emerg Infect Dis. 2004. PMID: 15078592 Free PMC article.
-
Investigation of the second wave (phase 2) of severe acute respiratory syndrome (SARS) in Toronto, Canada. What happened?Can Commun Dis Rep. 2008 Feb;34(2):1-11. Can Commun Dis Rep. 2008. PMID: 18404809 English, French.
-
Severe acute respiratory syndrome (SARS) - an emerging infection of the 21st century.J Formos Med Assoc. 2003 Dec;102(12):825-39. J Formos Med Assoc. 2003. PMID: 14976561 Review.
-
Severe acute respiratory syndrome (SARS)--paradigm of an emerging viral infection.J Clin Virol. 2004 Jan;29(1):13-22. doi: 10.1016/j.jcv.2003.09.011. J Clin Virol. 2004. PMID: 14675864 Free PMC article. Review.
Cited by
-
A Case of Novel Corona Virus With Three Negative Nasopharyngeal Swabbings.Cureus. 2020 May 24;12(5):e8266. doi: 10.7759/cureus.8266. Cureus. 2020. PMID: 32596083 Free PMC article.
-
Identification of single-chain antibody fragments specific against SARS-associated coronavirus from phage-displayed antibody library.Biochem Biophys Res Commun. 2005 Apr 8;329(2):437-44. doi: 10.1016/j.bbrc.2005.02.003. Biochem Biophys Res Commun. 2005. PMID: 15737606 Free PMC article.
-
Long-term SARS coronavirus excretion from patient cohort, China.Emerg Infect Dis. 2004 Oct;10(10):1841-3. doi: 10.3201/eid1010.040297. Emerg Infect Dis. 2004. PMID: 15504274 Free PMC article.
-
SARS-CoV-2 Testing.Am J Clin Pathol. 2020 May 5;153(6):706-708. doi: 10.1093/ajcp/aqaa052. Am J Clin Pathol. 2020. PMID: 32227199 Free PMC article. No abstract available.
-
Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community-acquired pneumonia?Clin Infect Dis. 2005 Apr 15;40(8):1079-86. doi: 10.1086/428577. Epub 2005 Mar 16. Clin Infect Dis. 2005. PMID: 15791504 Free PMC article.
References
-
- SARS situation update and continuing national surveillance. Ottawa: Health Canada; 2003. Available: www.hc-sc.gc.ca/pphb-dgspsp/sars-sras/cn-cc/numbers.html (updated 2003 Sept 5; accessed 2003 Nov 24).
-
- Drosten C, Gunther S, Preiser W, van der WS, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348(20):1967-76. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348(20):1953-66. - PubMed
-
- Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med 2003;348(20):1995-2005. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
