Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells
- PMID: 14709546
- PMCID: PMC2171967
- DOI: 10.1083/jcb.200306112
Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells
Abstract
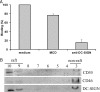
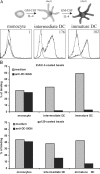
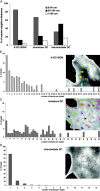
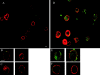
The C-type lectin dendritic cell (DC)-specific intercellular adhesion molecule grabbing non-integrin (DC-SIGN; CD209) facilitates binding and internalization of several viruses, including HIV-1, on DCs, but the underlying mechanism for being such an efficient phagocytic pathogen-recognition receptor is poorly understood. By high resolution electron microscopy, we demonstrate a direct relation between DC-SIGN function as viral receptor and its microlocalization on the plasma membrane. During development of human monocyte-derived DCs, DC-SIGN becomes organized in well-defined microdomains, with an average diameter of 200 nm. Biochemical experiments and confocal microscopy indicate that DC-SIGN microdomains reside within lipid rafts. Finally, we show that the organization of DC-SIGN in microdomains on the plasma membrane is important for binding and internalization of virus particles, suggesting that these multimolecular assemblies of DC-SIGN act as a docking site for pathogens like HIV-1 to invade the host.
Figures









References
-
- Akira, S. 2003. Mammalians Toll-like receptors. Curr. Opin. Immunol. 15:5–11. - PubMed
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Brown, D.A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. - PubMed
-
- Cambi, A., K. Gijzen, I.J.M. de Vries, R. Torensma, B. Joosten, G.J. Adema, M.G. Netea, B.J. Kullberg, L. Romani, and C.G. Figdor. 2003. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 33:532–538. - PubMed

